Detection of carbon and hydrogen:
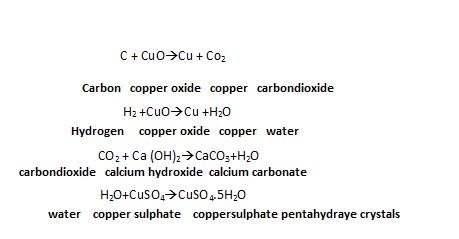
They are detected by heating the compound wit copper oxide. Carbon present in the compound is oxidised to Carbon-dioxide (tested with Lime water) and Hydrogen to Water(tested with anhydrous Copper sulphate ).
Detection of other elements : Nitrogen ,Sulphur, Halogen ,Phosphorous presence in organic compound are detected by Lasagne’s test .The elements present in the compound are converted into covalent form in to an ionic form by fusing the compound with Sodium metal .

Test for Nitrogen : The Sodium fusion extract is boiled with Ferrous Sulphate and then acidified with concentrated Sulphuric acid . The formation of Prussian Blue colour confirms the presence of Nitrogen .

Test for Sulphur : The Sodium fusion extract is acidified with Acetic acid with Lead acetate is added to it .A Black precipitate of Lead Sulphide indicates the presence of Sulphur.
S2- + Pb2+ –> PbS
Sulphide Lead Lead Sulphide
On treating Sodium fusion extract with Sodium Nitro Prusside, the Violet colour appears that confirms Sulphur .
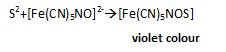
Test for halogens: The Sodium fusion extract is acidified with Nitric acid and then treated with Silver Nitrate.
- A White precipitate soluble in Ammonium hydroxide shows the presence of Chlorine.
- A Yellow precipitate soluble in Ammonium hydroxide shows the presence of Bromine.
- A Yellow precipitate not soluble in Ammonium hydroxide shows the presence of Iodine.
- X– + Ag+ –> AgX
- Halide Silver ion SilverHalide
Test for phosphorous: The compound is heated with Sodium Peroxide. The Phosphorus gets oxidised to Phosphate .Then the solution is boiled with Nitric acid and then treated with Ammonium mol bate .A Yellow precipitate indicates phosphorous.
Quantitative analysis
- Carbon and hydrogen: Both of them are estimated in one experiment. A known mass of Organic compound is burnt in presence of excess of Oxygen and Copper (ii)oxide . Carbon and Hydrogen both are oxidised to form Carbon dioxide and Water.
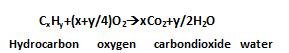
The mass of Water produced is determined by passing a mixture through U-tube containing anhydrous Calcium chloride. Carbon dioxide is absorbed in another U-tube containing solution of Potassium hydroxide. The increase in mass of calcium chloride and Potassium hydroxide gives the amount of Carbon dioxide and water .From it the percentage of C and H can be calculated.
Let the mass of organic compound = mg
Mass of water = m1
Mass of carbodioxide =m2
% of C = (12 x m2 x 100)/(44x)m
% of H = (2 x m1 x 100)/(18x)m
Nitrogen : There are two methods for estimation of Nitrogen :
- Dumas method
- Kjeldahl’s method
Dumas method: In this Nitrogen containing compound is heated with Copper oxide in an atmosphere of Carbon dioxide,yields free Nitrogen in addition to Carbon dioxide and water .
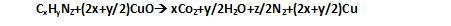
The Nitrogen oxides formed are reduced to Nitrogen by passing the gaseous mixture over a heated Copper gauze. The mixture of gases so produced is collected over aqueous Potassium hydroxide which absorbs Carbon dioxide. Nitrogen is collected in the upper part of graduated tube.
Volume of N at STP = (P1V1 x 273)/(760 x T1)
Kjeldahl’s method: The compound containing Nitrogen is heated with concentrated Sulphuric acid . The Nitrogen in the compound gets converted to Ammonium sulphate. The resulting acid mixture is then heated with excess Sodium hydroxide .The liberated Ammonia gas is absorbed in excess of standard solution of Sulphuric acid. The amount of Ammonia produced is determined by estimating the amount of Sulphuric acid consumed in the reaction.

- Halogens : For them we have Carious method. A known mass of organic compound is heated with fuming Nitric acid in the presence of Silver nitrate contained in a hard glass tube known as Carious tube in a furnace.
The Carbon and Hydrogen present in compound are oxidised to Carbon dioxide and water. The Halogens present forms the Silver halide .It is filtered, washed, dried and weighed.
Let the mass of organic compound = mg
Mass of AgX = m1 g
1 mole of AgX contains 1 mole of X
% of Halogen = (Atomic mass of X x m1 x 100)/(molecular mass of AgX) x m
- Sulphur: A known mass of organic compound is heated in Carious tube with Sodium peroxide or fuming Nitric acid. Sulphur present is oxidised to Sulphuric acid .It is precipitated with Barium sulphate by adding excess of Barium chloride solution in water .The precipitate is filtered ,washed and dried and weighed .
Let the mass of organic compound = mg
Let the mass of ammonium phosphor molybate = m1 g
% of Sulphur = (32 x m1 x 100)/(233) x m
- Phosphorous: A known mass of organic compound is heated with fuming Nitric acid. As a result Phosphorous gets oxidised to Phosphoric acid. It is then precipitated with Ammonium molybdate .
Let the mass of organic compound = mg
Let the mass of ammonium phosphor molybate = m1 g
% of Phosphorous = (62 x m1 x 100)/(222) x m
- Oxygen: A definite mass of organic compound is decomposed in stream of Nitrogen gas .The mixture of gaseous products containing oxygen is passed over red hot coke when all the oxygen present is converted into Carbon monoxide .Then this mixture is passed over Iodine pent oxide, when CO is oxidised to Carbon dioxide producing Iodine.