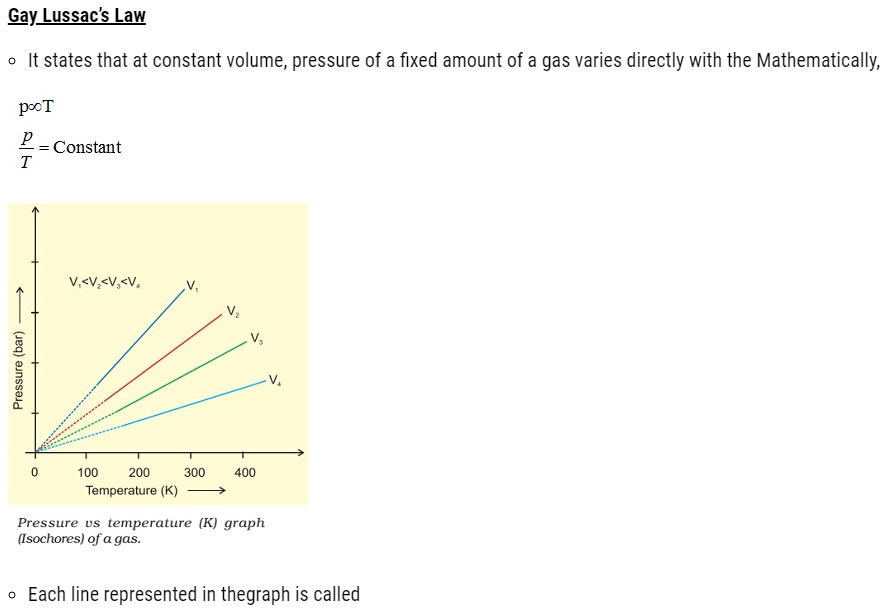
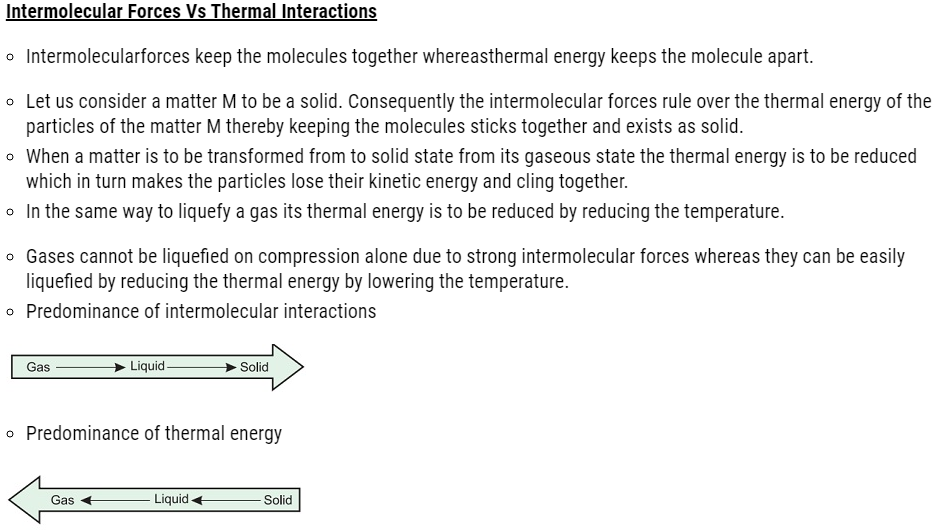
About Lesson




Charles’ Law
- It states that for a fixedmass of a gas at constant pressure, volumeof a gas increases on increasing temperatureand decreases on cooling. Mathematically,
V/T = k
- Let V1 = initial volume
T1 = initial temperature
After performing the experiment let V2 = final volume
T2 = final temperature
Hence, V1 ÷ T1 = k
Again V2 ÷ T2 = k
Since k = k, it can be concluded
V1 / T1 = V2 / T2

Solution: According to Charles’s law
V2/T2 = V1/ T1
Volume of gas expelled out = V2 – V1 ……………….. (I)
Fraction of gas expelled out = (V2 – V1) / V2 = 1- (V1/ V2) ……………. (II)
From equation (I) V1/ V2 = T1/ T2 ………….. (III)
Putting the values of (III) in (II)
Fraction of the air expelled out = 1- T1/ T2 = (T2 – T1)/ T2
= 750- 300 /750 = 0.6
Fraction of air expelled out is 0.6 or 3/5 th