Acids, bases and salts
The Substances that get dissociated are broadly classified into three types:
- Acids
- Bases
- Salts
Acids: Are those that are sour and turn blue litmus red.
Bases: Are those that are soapy in touch and turn red litmus blue.
Salts: Are those that are formed when acid and base react.
But this information is not relevant or sufficient to classify substances. So, we have different concepts and attempts in order to classify the substances.
1st concept: Arrhenius concept of acids –bases
According to Arrhenius:
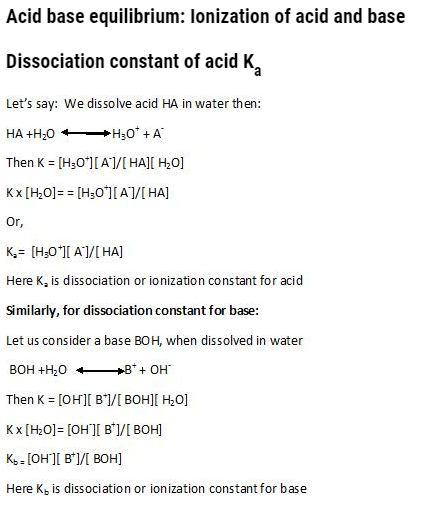
Acids are those substances which when dissolved in water produce hydrogen ions.
Example: HCl àH+ + Cl–
Later on, it was said that this hydrogen ions combines with water to form hydronium ion i.e. H3o+
Bases: Are those which when dissolved in water, release hydroxide ions.
Example: NaOHàNa+ + OH–
Later on, it was seen that there are few substances that do not release hydrogen or hydroxide ion. But, still they behave as acids or bases .So, another concepts were introduced.
2nd Concept: Bronsted Lowry concept
According to it:
Acids: Which give hydrogen ion in solution (as they are proton donors).
Example: HClàH+ + Cl–
Bases: Are those which are proton acceptors.
Example: NH3 + H+ àNH4+
Conjugate acid –base concept: If any strong acid dissociates, it will produce weak conjugate base that is:
HClàH+ + Cl–
(HCl is strong acid)(Cl– is conjugate base)
Or we can say :
Acid – H+ àconjugate base
Base + H+à conjugate acid
There are certain substances that behave as acids as well as bases, they are called Amphoteric substances.
Example: H2O + H+àH3O+
(Base) (Conjugate acid)
H2OàH+ + OH–
(Acid) (Conjugate base)
3rd concept: Lewis concept
According to him:
Acids: They are those which accept a pair of electron.
For example: substances that falls in this category:
- Cations like ammonium ion etc
- All neutral electron deficient compounds like BF3, AlCl3 etc
- All those which have empty d orbital like SF4 etc
- All those different elements with different electro-negativities but bonded with multiple bonds like carbon dioxide etc.
Bases: Are those that are electron donors. They are of two types:
- Anions like chloride ions etc
- All neutral species having lone pair of electrons like water etc
Limitation of this concept: There are many substances which are Lewis bases and acids, but they may not be Arrhenius acid or base or Lowry acid or base.

Please note:
- Acid + water à concentration of hydronium ion is more than hydroxide ion.
- Base + water à concentration of hydronium ion is less than hydroxide ion.
- Pure water à concentration of hydronium ion and hydroxide ion is same.
Expressing Hydrogen ion concentration: pH scale
To know nature of any substances, we use pH scale.
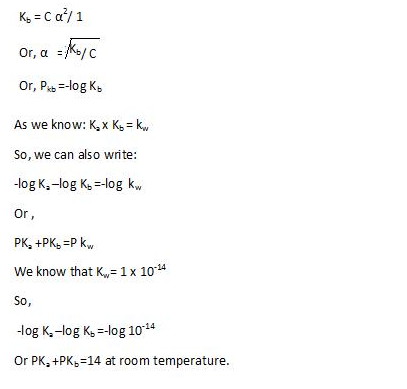
pH of solution: It is defined as negative of logarithm of hydrogen ion concentration.
Mathematically:- pH = – log[H3O]+
pH is a measure of acidic or basic strength of any solution.
pH scale is given below:
Scale is just like a number line on which there is a reading ranging from 0 to 14.
- Up to 7: acidic
- At 7: neutral
- Above 7 : basic
- All solutions having pH 4 to 7 : weak acid
- pH 2 to 4 : moderately acidic
- pH 7: neutral
- pH less than 2: strong acid
- pH =14: strong base
- pH 7 to 9: weak base
Calculation of pH:
pH = – log[H3O]+