NCERT Class 11 Chemistry Texbook Chapter 14 Solved Questions ( Environmental Chemistry )
Question 1. Define environmental chemistry?
Answer: Environmental Chemistry is the branch of science which deals with the chemical changes in the environment. It includes our surroundings such as air, water, soil, forest etc.
Question 2. Explain the tropospheric pollution in 100 words?
Answer: Tropospheric pollution occurs due to the presence of undesirable substance in air. These may be the solid or gaseous pollutants.
- Gaseous Air Pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
- Particulate Pollutants: These are dust, mist, fumes, and smog etc.
Question 3. Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Answer: Carbon monoxide combines with haemoglobin to form a very stable compound known as carboxyhaemoglobin when its concentration in blood reaches 3-4%, the oxygen carrying capacity of the blood is greatly reduced. This results into headache, nervousness and sometimes death of the person. On the other hand CO2 does not combine with haemoglobin and hence is less harmful than CO.
Question 4. Which gases are responsible for greenhouse effect? List some of them.
Answer: CO2 is mainly responsible for greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapours, CFCs and Ozone.
Question 5. Statues and monuments in India are affected by acid rain. How?
Answer: This is mainly due to the large number of industries and power plants in the nearby areas. Acid rain has vapours of sulphuric acid dissolved in it. When it comes in contact with various statues or monuments, the acid reacts chemically with calcium carbonate.
CaCO3 + H2SO4 ——–> CaSO4 + H2O + CO2
Question 6. What is smog? How is classical smog different from photochemical smog?
Answer: The word smog is a combination of smoke and fog. It is a type of air pollution that occurs in many cities throughout the world. Classical smog occurs in cool humid climate. It is also called as reducing smog.Whereas photochemical smog occurs in warm and dry sunny climate. It has high concentration of oxidising agents and therefore, it is also called as oxidising smog.
Question 7. Write down the reactions involved during the formation of photochemical smog.
Answer: Mechanism of formation of photochemical smog:
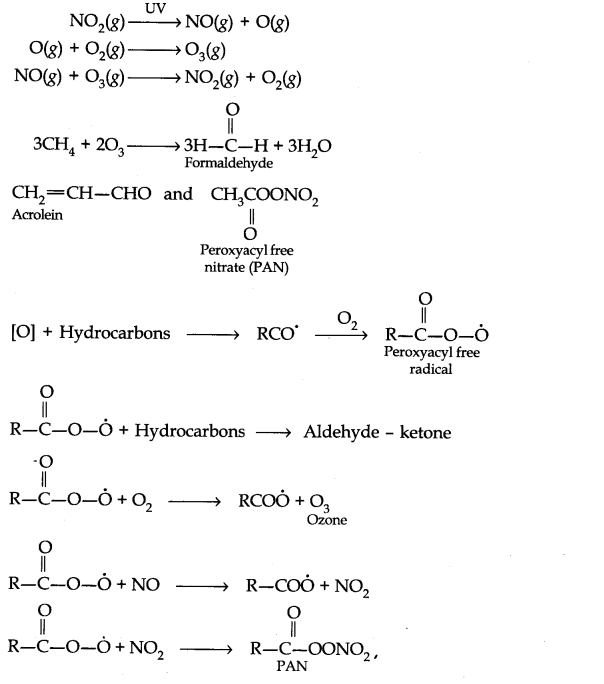
Question 8. What are the harmful effects of photochemical smog and how can they be controlled?
Answer: Harmful effects of photochemical smog:
- Their’high concentration causes headache, chest pain and dryness of the throat.
- Ozone and PAN act as powerful eye irritants.
- Photochemical smog leads to cracking of rubber and extensive damage to plant life.
- It causes corrosion of metals, stones, building materials, and painted surface etc.
Control:
- Use of catalytic converter in automobiles prevents the release of nitrogen dioxide and hydrocarbons to the atmosphere.
- Pinus, juniparus, quercus, pyrus etc. can metabolise nitrogen dioxide thus their plantation could help to some extent.
Question 9. What are the reactions involved for ozone layer depletion in the stratosphere?
Answer: The reaction can be shown as follows:
CF2Cl2(g) + UV ——-> Cl(g) + CF2Cl(g)
Cl(g) + O3(g) ———-> ClO (g) + O2(g)
ClO(g) + O(g) ———> Cl + O2(g)
Question 10. What do you mean by ozone hole? What are its consequences?
Answer: Depletion of ozone layer creates some sort of holes in the blanket of ozone which
surround us, this is known as ozone hole.
- With the depletion of the ozone layer, UV radiation filters into the troposphere which leads to aging of skin, cataract, sunburn, skin cancer etc.
- By killing many of the phytoplanktons, it can damage the fish productivity.
- Evaporation rate increases through the surface and stomata of leaves which can decrease the moisture content of the soil.
Question 11. What are the major causes of water pollution? Explain.
Answer: Causes of water pollution:
- Pathogens: Pathogens include bacteria and other microorganisms that enter water from domestic sewage and animal excreta.
Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis ,
which cause gastrointestinal diseases. - Organic wastes: Organic wastes when added to water, as these are biodegradable, bacteria decomposes organic matter and consume dissolved oxygen in water. When the concentration of dissolved oxygen of water is below 6 ppm, the growth of fish gets inhibited. Breakdown of the organic wastes by anaerobic bacteria produces chemicals that have a foul smell and are harmful to human health.
- Chemical pollutants: Some inorganic chemicals as an industrial wastes dissolve in water like cadmium, mercury nickel etc. These metals are dangerous to humans and other animals. These metals can damage kidneys and central nervous system,lever etc. Petroleum products pollute many sources of water.
Question 12. What do you mean by Biochemical Oxygen Demand (BOD)?
Answer: The amount of oxygen required by bacteria to breakdown the organic matter present in a certain volume of a sample of water is called Biochemical Oxygen Demand (BOD).
Question 13. What are pesticides and herbicides? Explain giving examples.
Answer: Pesticides are the chemical compounds used in agriculture to control the damages caused by insects, rodents, weeds and various crop diseases.
Example: Aldrin, Dilldrin, B.H.C etc.
Herbicides: These are the chemicals used to control weeds.
Example: Triazines.
Question 14. What do you mean by green chemistry? How will it help in decreasing environmental pollution ?
Answer: Green chemistry is a way of thinking and is about utilising the existing knowledge and principles of chemistry and other sciences to reduce the adverse effect of pollution.
For example:
- Automobile engines have been fitted with catalytic converters which prevent the release of the vapours of hydrocarbons and oxides of nitrogen into acrolein and peroxyacetyl nitrate.
- CO2 has replaced CFCs as blowing agents in the manufacture of polystyrene foam sheets.
Question 15. What would have happened if the greenhouse gases were totally missing in the earth’s atmosphere? Discuss.
Answer: The solar energy radiated back from the earth surface is absorbed by the green house gases. (CO2, CH4, O3, CFCs) are present near the earth’s surface.
They heat up the atmosphere near the earth’s surface and keep it warm. As a result of these, there is growth of vegetation which supports the life. In the absence of this effect, there will be no life of both plant and animal on the surface of the earth.
Question 16. A large number offish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill.
Answer: Excessive phytoplankton (organic pollutants such as leaves, grass trash etc.) present in water are biodegradable. Bacteria decomposes these organic matters in water. During this process when large number of bacteria decomposes these organic matters, they-consume the dissolved oxygen in water. When the level of dissolved oxygen falls below 6 ppm the fish cannot survive.
Question 17. How can domestic waste be used as manure?
Answer: Domestic waste consists of biodegradable waste which can be converted into manure by suitable method.
Question 18. For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odour, flies and recycling of wastes for a good produce.
Answer: The compost producing pit should be kept covered so that flies cannot make entry into it and bad odour is minimized.
The waste materials which are non-biodegradable like glasses, plastic bags, polybags, must be handed over to the vendors who can send them to the recycling plants.