PART 1
PART 2
Introduction
As we have already studied about Daltons Atomic Theory of Matter, and later it was concluded that Atom is made up of 3 sub -atomic particles. The particles are Electron, Proton and Neutron. In this chapter ,we will study about different attempts made to explain atomic models .The phenomenon that leads to development of dual nature of Light and Matter .Also, we will deal with the methods to be followed while writing electronic configurations, and much more . Let us see that first how these fundamental particles were discovered, and how they are arranged in structure of Atom.
DISCOVERY OF ELECTRON:
The Electron was discovered by J.J Thomson by conducting a Cathode ray tube experiment.
For the experiment he used Crooke’s tube, which was 60cm long glass tube and had a small tube attached. To this small tube vacuum pump was attached, it also had two metal plates which were connected to battery by wires.

When current was passed under same conditions it also started glowing green. This confirmed that under those conditions some rays were emitted through cathode, and were travelling towards anode. Those rays were called as cathode rays and found to consist of negatively charged particles called electron.
Properties of cathode rays
They are found to travel in straight line. This property was concluded by performing an activity where the object was placed in their path. When they strike it ,they casted the shadow of the object as shown below:
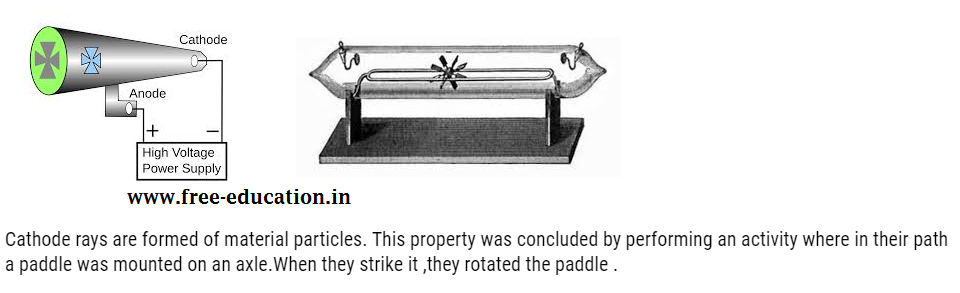

From the above properties and experiment it was concluded that cathode rays are made up of negatively charged particles called electron.
Further experiments were carried out in order to know the charge and mass of electron. It was found to be:
- Charge :1.6 x 10-19C
- Mass 9.1 x 10-31kg
We can define electron as:
- “A fundamental particle that is negatively charge of magnitude 1.6 x 10-19C and mass equal to 9.1 x 10-31