About Lesson


Trends in the modern periodic table ( Classification of Elements )
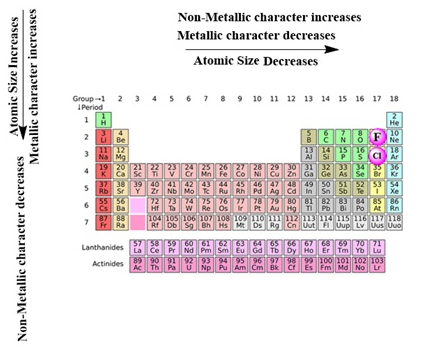
- The number of shells increases on going down the group.
- The number of valence shell electrons increases with the increase in atomic number on moving from left to right in a period with each period marking the filling of a new electronic shell.
- Atomic size decreases in moving from left to right along a period due to an increase in nuclear charge pulling the electrons closer to the nucleus.
- Addition of new shells down the group increases the distance between the outermost electrons and the nucleus thereby increasing the atomic size down the group.
- Across a period effective nuclear charge acting on the valence shell electrons increases which decreases the tendency to lose electrons. Hence metallic character decreases and non-metallic character increases across a period.
- Down the group, the effective nuclear charge decreases which increases the tendency to lose electrons. Hence metallic character increases and non-metallic character decreases down a group.