NCERT Solutions for Class 11 Chemistry Chapter 9 Very Short Answer Type Questions
Question 1. Which isotope of hydrogen is radioactive?
Answer: Tritium.
Question 2. What is the importance of heavy water with regard to nuclear power generation ?
Answer: It is used as a moderator and helps to control the nuclear reaction.
Question 3. What is zeolite?
Answer: It is hydrated sodium aluminium silicate, Na2Al2Si2O8.xH2O.

Question 7. Give two advantages of using hydrogen over gasoline as a fuel.
Answer:
- High heat of combustion
- It is pollution free.
Question 8. What type of elements form interstitial hydrides?
Answer: d-block and f-block elements.
Question 9. What is perhydrol?
Answer: Perhydrol is the trade name of H2O2. It is used as an antiseptic.
Question 10. What is meant by hard water?
Answer: Water which does not produce lathers with soap is known as hard water. Hardness is due to the presence of bicarbonates, sulphates and chlorides of Ca2+ and Mg2+ .
Question 11. Which gas is evolved when Mg3N2 (Magnesium nitride) is treated with H2O? Give chemical reaction.
Answer: NH3 gas is evolved.
Mg3N2 + 6H2O ———–> 3Mg(OH)2 + 2NH3
Question 12. Which compounds cause temporary hardness of water?
Answer: Ca(HCO3)2 and Mg (HCO3)2
Question 13. Which isotope of hydrogen does not have neutron ?
Answer: 11H does not have neutron. It is called protium.
Question 14. Name a substance which can oxidise H2O2
Answer: Acidified KMnO4.
Question 15. Which type of hydrides are generally non-stoichiometric in nature?
Answer: Interstitial hydrides are non-stoichiometric in nature.
Question 16. What is the cause of bleaching action of H2O2?
Answer: H2O2(l) ———-> H2O(l) + O(g)
Nascent oxygen produced is responsible for bleaching action.
Question 17. What is the use of hydrogen in the manufacture of Vanaspati Ghee?
Answer: H2 is used as reducing agent to convert vegetable oil into vegetable ghee.
Question 18. Name the phenomenon of adsorption of hydrogen on metal surface.
Answer: Occlusion.
NCERT Solutions for Class 11 Chemistry Chapter 9 Short Answer Type Questions
Question 1. Show how H2O2 junctions both as a reducing and as an oxidising agent.
Answer: As oxidising agent.
2I– + H2O2 + 2H+ ———> I2 + 2H2O
As reducing agent.
H2O2 + Ag2O ———-> 2Ag + H2O+ O2
Question 2. What are interstitial hydrides? Give two examples.
Answer: Many transition and inner-transition metals absorb hydrogen into the interstices of their lattices to yield metal like hydrides also called the interstitial hydrides. These hydrides are generally non stoichiometric and their composition vary with temperature and pressure.
For example,TiH1.73, CeH2.7


Question 6.(a) How is dihydrogen prepared from water by using a reducing agent?
(b) Give the industrial use of dihydrogen which depends upon heat liberated when it bums.
Answer: (a) Dihydrogen is prepared from water by the action of alkali metals like Na and K which is a strong reducing agent.
2Na + 2H2O ———-> 2NaOH + H2
2K + 2H2O ———> 2KOH + H2
(b)For welding purposes.
H2(g) + 1\2 O2(E) ————> H2O (g) + heat
Question 7. Water molecule is bent, not linear. Explain?
Answer: In water molecule, O is SP3 hybridized. Due to stronger lone pair-lone pair repulsion than bond pair-bond pair repulsions, the HOH bond angle decreases from 109.5° to 104.5°. Thus water is bent molecule.
Question 8. Account for the following:
(i) dihydrogen gas is not preferred in balloons.
(ii) Cone. H2S04 cannot be used for drying H2.
Answer: (i) Dihydrogen is the lighest gas but due to its highly combustible nature it is not preferred in balloons.
(ii) Cone. H2S04 on absorbing H2O forms moist H2 produces so much heat that hydrogen catches fires.
Question 9. Calculate the volume strength of a 3% solution of H2O2
Answer: 100 ml of H2O2 solution contain H2O2 = 3g.
.’. 1000 ml of H2O2 solution will contain = 3/100x 1000 = 30g
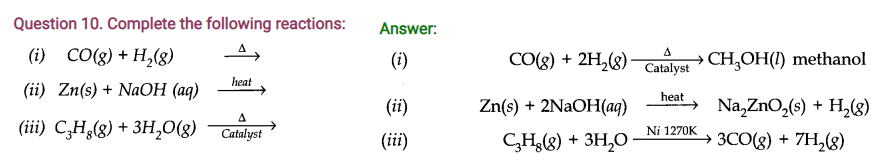
NCERT Solutions for Class 11 Chemistry Chapter 9 HOTS Questions
Question 1. Account for the following:
(a) Can phosphorus with electronic configuration 3s2 3p3 form PH5?
(b) Water is responsible for moderation of body temperature. How?
(c) Hard water is not suitable for boilers as well as for laundary.
Answer: (a) High ∆aH value of dihydrogen and less negative value of ∆eg H of hydrogen do not favour to exhibit highest oxidation state of P and consequently the formation of PH5, although P exhibit +3, +5 oxidation states.
(b) Because of high heat of vapourisation and high heat capacity.
(c) Hard water form precipitate with soap and deposition of salts in the form of scales.
Question 2. Can we use concentrated sulphuric acid and pure zinc in the preparation of dihydrogen?Write the chemical reactions to show the amphoteric nature of water. Why is hydrogen peroxide stored in wax-lined plastic coloured bottles ?
Answer: (a) Cone. H2S04 cannot be used because it acts as an oxidizing agent also and gets reduced to SO2.
Zn + 2H2S04 (Cone.) ———> ZnS04+ 2H2O + SO2
Pure Zn is not used because it is non-porous and reaction will be slow. The impurities in Zn help in constitute of electrochemical couple and speed up reaction.
(b) Water is amphoteric in nature and it behaves both as an acid as well as base. With acids stronger than itself (e.g., H2S) it behaves as a base and with bases stronger than itself (e.g., NH3) it acts as an acid.
(i) As a base: H2O(I) + H2S(aq) ——-> H3O(aq) + HS–(aq)
(ii) As an acid: H2O(I) + NH3(aq) ———> OH–(aq) + NH4+(aq)
(c) The decomposition of H2O2 occurs readily in the presence of rough surface (acting as catalyst). It is also decomposed by exposure of light. Therefore, waxlined smooth surface and coloured bottles retard the decomposition of H2O2.