Chapter – 13 Hydrocarbons Part- 1 || Class 11th Chemistry
Chapter – 13 Hydrocarbons Part- 2 || Class 11th Chemistry
Chapter – 13 Hydrocarbons Part- 3 || Class 11th Chemistry
Introduction ( Hydrocarbons Notes and Solution Class 11 Chemistry )
These are compounds of Hydrogen and carbon and we can derive other compounds from it by substituting Hydrogen with some other groups.
They are generally called Fuel Compounds:
Like CH₄ – is used as Biogas.
Butane -is used in LPG.
Hydrocarbons -are used in Kerosene, Diesel, Gasoline etc.
Classification of hydrocarbons
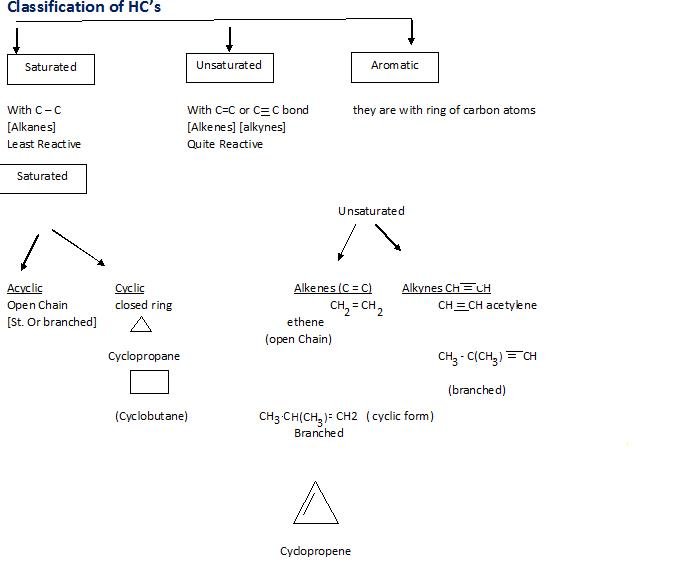
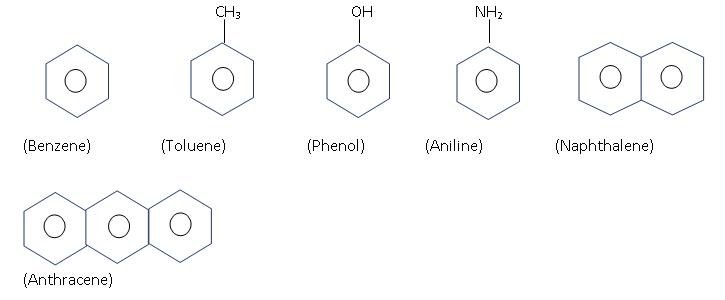
Aromatic: They have at least one Benzene ring.
Alkanes
They are saturated Hydrocarbons with General Formulaà Cn H2n +2
Where “n” = number of atoms in a chain.
These alkanes may be open chain like CH4, C2H6, C3H8, C4H10 , C5H12
These can be cyclic alkanes with formula Cn H2n like Cyclopropane etc.
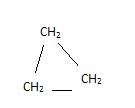
(Cyclopropane)
Introduction of alkanes
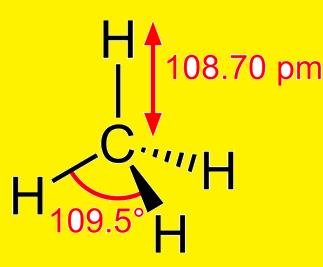
Structure of Alkanes – In them there is Sp3 hybridisation as explained below:
Atomic number of carbon = 6
C = 1s2 2s2 2p2
1s2, 2s1 2px1 2py1 2pz1 [ it is Tetravalent]
So, in CH4 (Methane) the structure is:
Nomenclature
While writing their IUPAC names the suffix used is “ane”.
For example: For writing name of CH4 the suffix used is “ane” that is “methane”.
Few more examples: like
- 2, 5 dimethyl hexane
- 3 ethyl – 2 , 2 dimethyl hexane
- Cyclohexane
- Methyl Cyclohexane
As you can clearly observe their names and see that their names end with “ane” so just by looking at their names we can make out they all are alkanes.
Isomerism is alkanes
Isomers: They are the compounds with same molecular formula but different Structural formula.
Alkanes: They show structural isomerism that is Chain isomerism is common in them.
In chain isomerism: In it the molecular formula is same, but the skeletal arrangement of atoms in chain is different.
Please note this that it is not shown by CH4, C2H6 and C3 H8 that is methane, ethane and propane. It starts with Butane as shown below:
