NCERT Solutions for Class 11 Chemistry Chapter 11 Very Short Answer Type Questions
Question 1. Why is boron used in nuclear reactions?
Answer: Because Boron can absorb neutrons.
Question 2. Why does boron form stable electron deficient compounds?
Answer: Boron has three electrons in its valence shell that boron show three electrons with other elements and form an electron-deficient compound.
Question 3. By giving a balanced equation show how B(OH)3 behaves as an acid in water.
Answer: B(OH)3 + 2HOH ———> [B(OH)4]– + H3O+.
Question 4. What is dry ice? Why is it so called?
Answer: Carbon dioxide can be obtained as a solid in the form of dry ice by allowing the liquified CO2 to expand rapidly.
Unlike ordinary ice it does not melt and hence does not wet the surface on which it is kept. Thus it is called dry ice.
Question 5. Name the element of group 14 which exhibits maximum tendency for catenation.
Answer: Carbon.
Question 6. What is the basic building unit of all silicates?
Answer: SiO44- is the basic unit of all silicates.
Question 7. Why do boron halides form addition compounds with NH3?
Answer: Boron halides are lewis acids and can accept a pair of electrons from amines to form addition product.
Question 8. What happens when NaBH4 reacts with iodine?
Answer: 2NaBH4 + I2 ———–> B2H6 + 2NaI + H2.
Question 9. Out of CCl4 and SiCl4 which one react with water and why?
Answer: Due to the presence of d-orbitals in Si, SiCl4 reacts with water. CCl4 does not react with water due to the absence of d-orbitals in C atom.
Question 10. Which oxide of carbon is regarded as anhydride of carbonic acid?
Answer: CO2 is regarded as anhydride of carbonic acid.
H2CO3 ————> H2O + CO2
Question 11. What happens when boric acid is heated?
Answer:

NCERT Solutions for Class 11 Chemistry Chapter 11 Short Answer Type Questions ( The p Block Elements )
Question 1. What is meant by catenation? Why does ‘C show the property of catenation to maximum extent?
Answer: It is the phenomenon of an atom to form a strong covalent bond with the atoms of itself. Carbon shares the property of catenation to maximum extent because it is small in size and can form Pπ-Pπmultiple bonds to itself.
Question 2. Give the chemical reactions as evidence for each of the following observations.
(i) Tin (II) is a reducing agent whereas lead (II) is not.
(ii) Gallium (I) undergoes disproportionation reaction.
Answer: (i) Due to inert pair effect Pb2+ is more stable than Pb4+. Whereas Sn4+ is more stable than Sn2+.
Thus Sn2+ is a good reducing agent and Pb2+ is not.
(ii) 3Ga+ ——–> 2Ga + Ga3+
This is because Ga3+ is more stable than Ga+.
Question 3. Describe two similarities and two dissimilarities between B and Al.
Answer: Similarities:
- Both have same number of valence electrons.
- Both have similar electronic configuration.
Dissimilarities:
- B is a non-metal where Al is a metal.
- B forms acidic oxide whereas Al forms amphoteric oxides.
Question 4. (a) What is general formula of silicons?
(b) How are linear silicons obtained?
Answer: (a) R2SiO
(b) Linear silicons are obtained by the hydrolysis of R2SiCl2 (chlorosilanes).

Question 7.Give reason.
(i) C and Si are always tetravalent but Ge, Sn, Pb show divalency.
(ii) Gallium has higher ionization enthalpy than Al. Explain.
Answer: (i) Ge, Sn and Pb show divalency due to inert pair effect, Pb2+ is more stable than Pb4+ .
(ii) Due to poor shielding effect of d-electrons in Ga, effective nuclear charge increases as compared to Al. Thus the I.E of Ga is higher than Al.
Question 8. Give reason why boron and aluminium tend to form covalent compounds.
Answer: Sum of the three ionization enthalpies of both the elements are very high. Thus they have no tendency to lose electrons to form ioriic compound. Instead they form covalent compounds.
NCERT Solutions for Class 11 Chemistry Chapter 11 Long Answer Type Questions ( The p Block Elements )
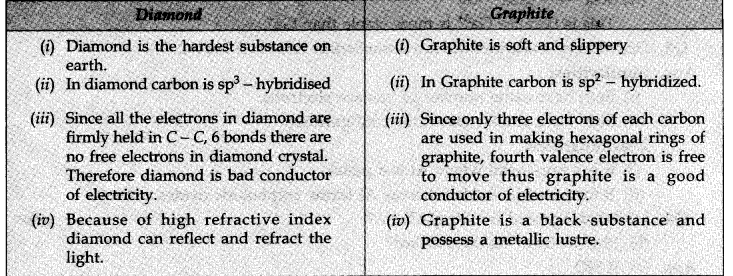
Question 1. Explain the differences in properties of diamond and graphite based upon their structures.
Answer:
Question 2. Give reasons:
(a) Why do Boron halides form an additional compounds with NH3?
(b) The tendency for catenation decreases down the group in Group 14.
(c) PbO2 is a stronger oxidizing agent than SnO2.
Answer: (a) It is because BX3 is an electron-deficient compound and NH3 is an electron-rich compound.
(b) As we move down group 14, the atomic size increases and thus the strength of the element decreases down the group thus the bond dissociation enthalpy decreases steadily consequently the tendency for catenation decreases down the group.
(c) PbO2 and SnO2 both are present in +4 oxidation state. But due to stronger inert pair effect Pb2+ ion is more stable than Sn2+ ion.
In other way Pb4+ ions is more easily reduced to Pb2+ions. Thus PbO2 acts as a stronger oxidising agent than SnO2.