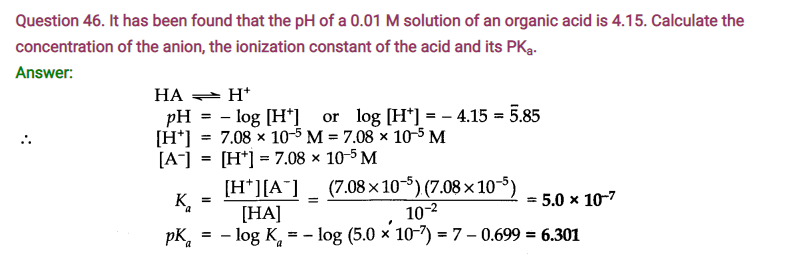
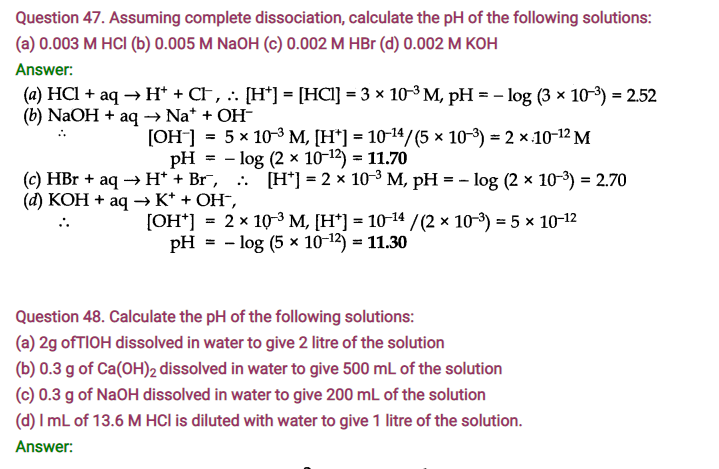
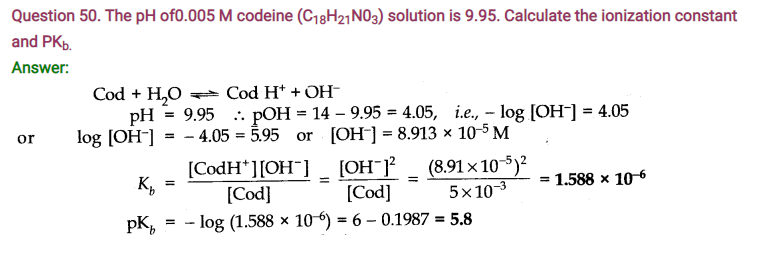
NCERT Solutions for Class 11 Chemistry Chapter 7 Equilibrium
NCERT TEXTBOOK QUESTIONS SOLVED (equilibrium class 11)
Question 1. A liquid is in equilibrium with its vapours in a sealed container at a fixed temperature. The volume of the container is suddenly increased, (i) What is the initial effect of the change on the vapour pressure? (ii) How do the rates of evaporation and condensation change initially? (iii) What happens when equilibrium is restored finally and what will be the final vapour pressure?
Answer: (i) On increasing the volume of the container, the vapour pressure will initially decrease because the same amount of vapours are now distributed over a larger space.
(ii) On increasing the volume of the container, the rate of evaporation will increase initially because now more space is available. Since the amount of the vapours per unit volume decrease on increasing the volume, therefore, the rate of condensation will decrease initially.
(iii) Finally, equilibrium will be restored when the rates of the forward and backward processes become equal. However, the vapour pressure will remain unchanged because it depends upon the temperature and not upon the volume of the container.


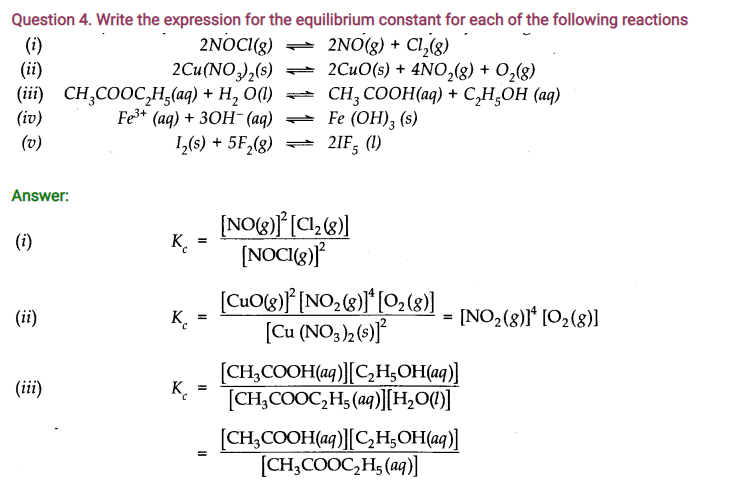

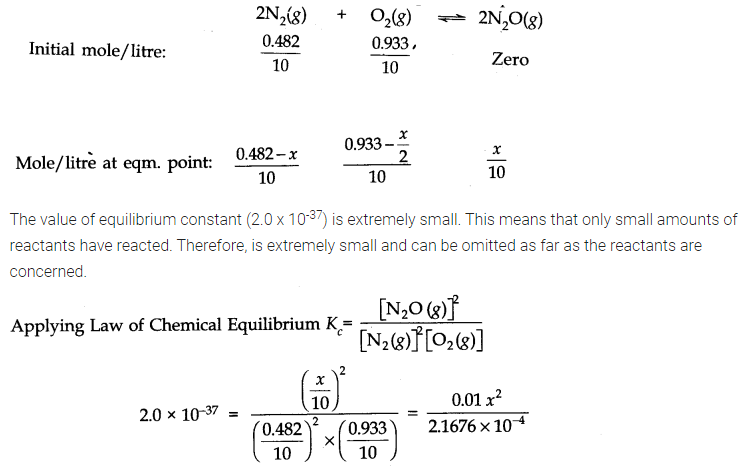
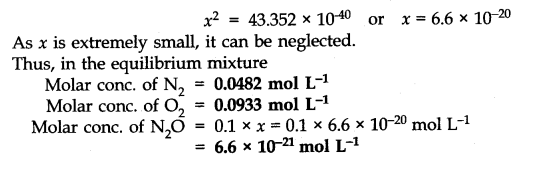




Answer: Number of moles of water originally present = 1 mol
Percentage of water reacted =40%
Number of moles of water reacted = 1 x 40/100 = 0.4 mol
Number of moles of water left = (1 – 0.4) = 0.6 mole According to the equation, 0.4 mole of water will react with 0.4 mole of carbon monoxide to form 0.4 mole of hydrogen and 0.4 mole of carbon dioxide.
Thus, the molar cone, per litre of the reactants and products before the reaction and at the equilibrium point are as follows:

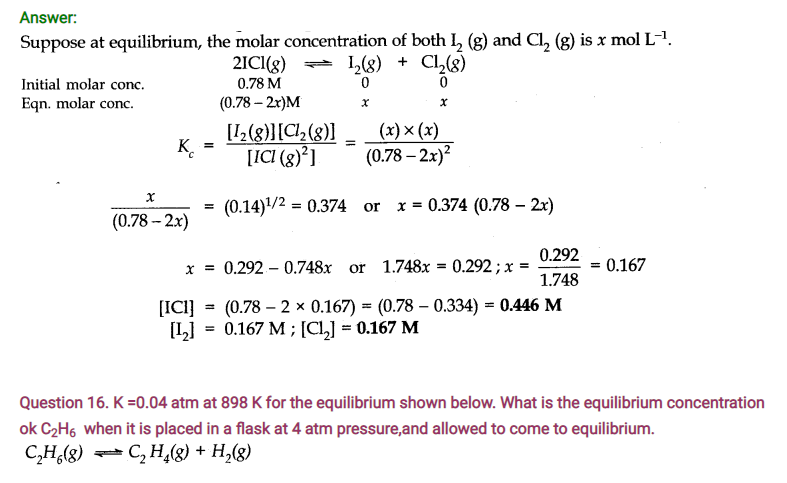

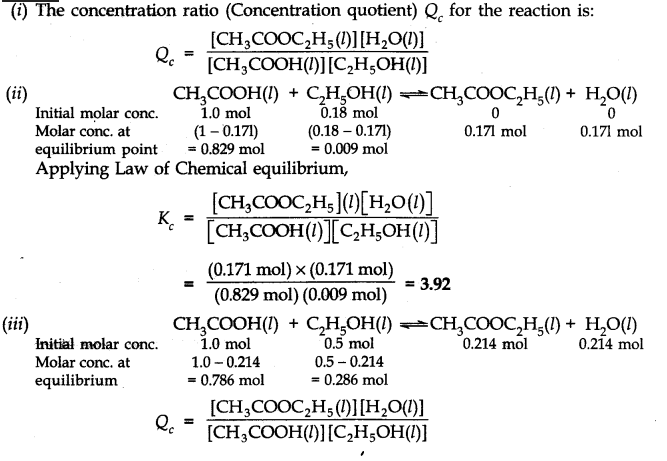
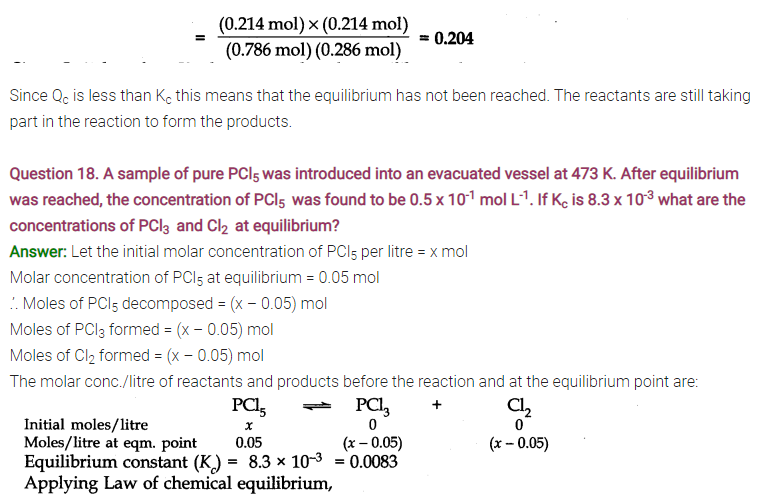
Question 17. The ester, ethyl acetate is formed by the reaction of ethanol and acetic acid and the equilibrium is represented as:
CH3COOH(l) + C2H5OH(l)——-> CH3COOC2H5(l) + H2O(l)
(i) Write the concentration ratio (concentration quotient) Q for this reaction. Note that water is not in excess and is not a solvent in this reaction.
(ii) At 293 K, if one starts with 1.000 mol of acetic acid and 0.180 mol of ethanol, there is 0.171 mol of ethyl acetate in the final equilibrium mixture. Calculate the equilibrium constant.
(iii) Starting mth 0.50 mol of ethanol and 1.0 mol of acetic acid and maintaining it at 293 K, 0.214 mol of ethyl acetate is found after some time. Has equilibrium been reached?
Answer:
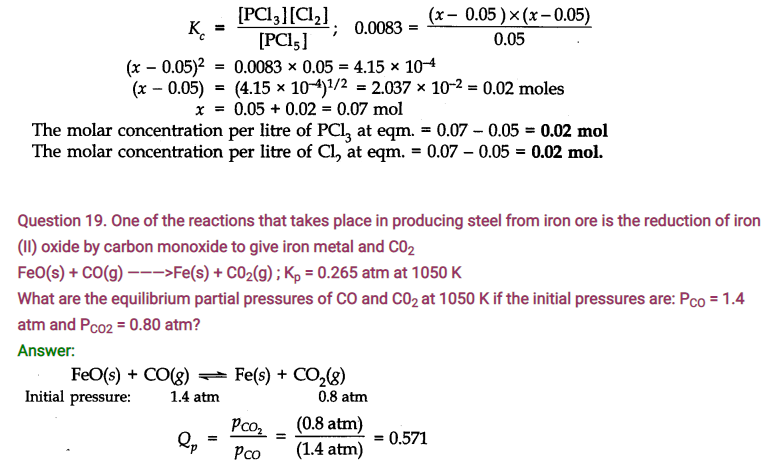
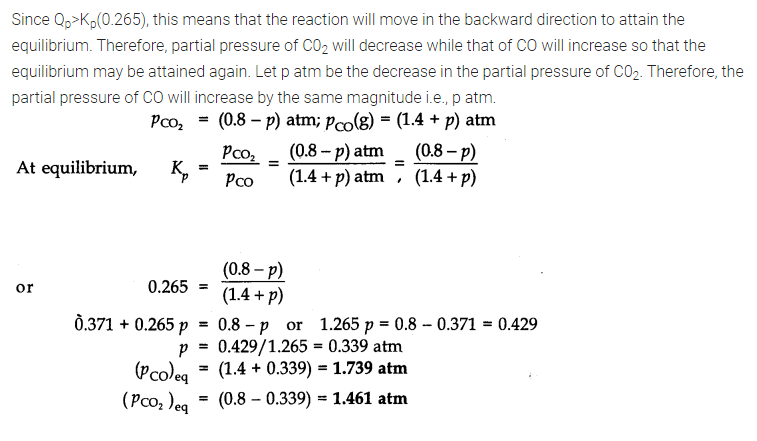

Question 21. Bromine monochloride (BrCl ) decomposes into bromine and chlorine and reaches the equilibrium:
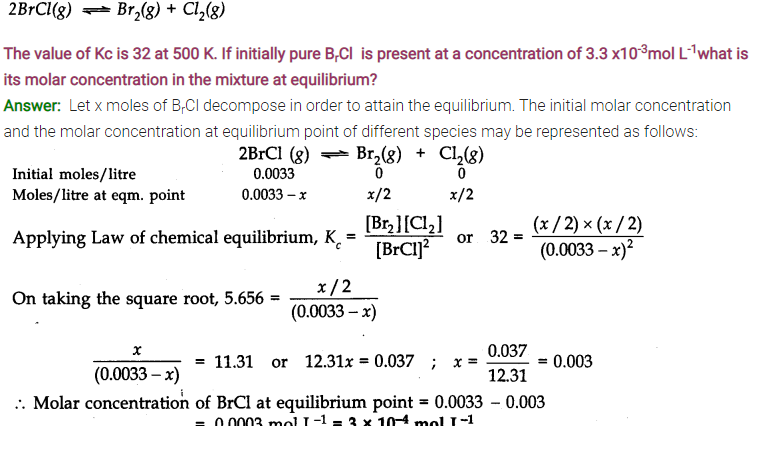

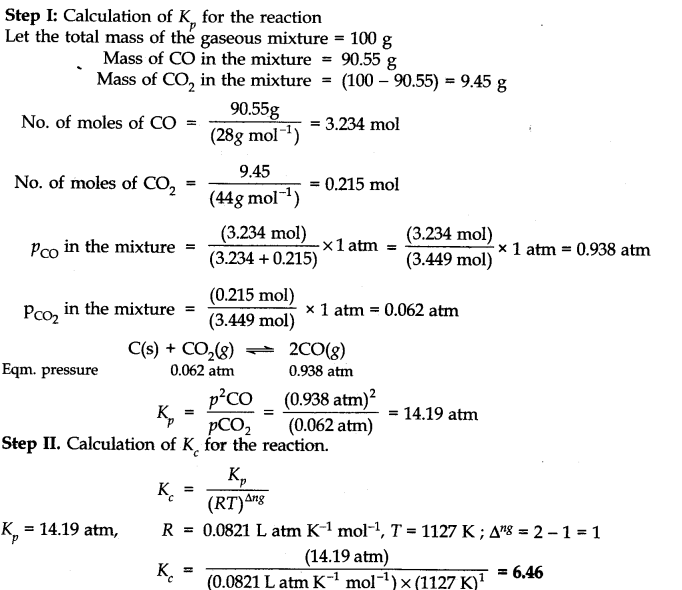

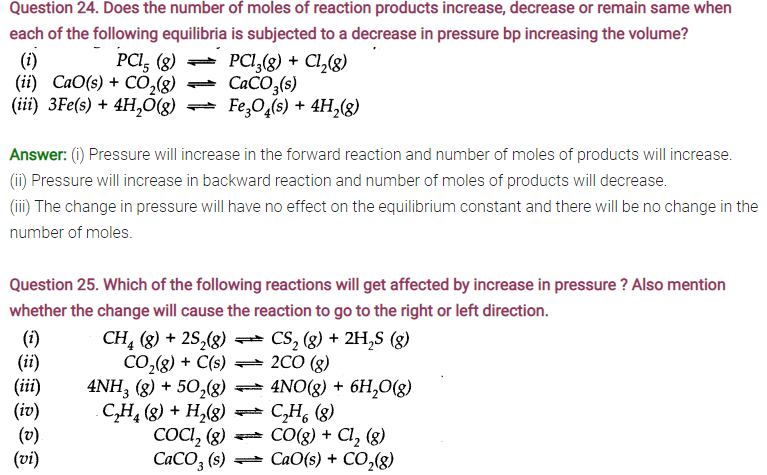
Answer: Only those reactions will be affected by increasing the pressure in which the number of moles of the gaseous reactants and products are different (np ≠ nr) (gaseous). With the exception of the reaction (1); all the reamaining five reactions will get affected by increasing the pressure. In general,
- The reaction will go to the left if np> nr.
- The reaction will go to the right if nr > np .
Keeping this in mind,
(i) Increase in pressure will not affect equilibrium because np = nr = 3.
(ii) Increase in pressure will favour backward reaction because np (2) > nr (1)
(iii) Increase in pressure will favour backward reaction because np (10) > nr (9)
(iv) Increase in pressure will favour forward reaction because np (1) < nr (2)
(v) Increase in pressure will favour backward reaction because np (2) > nr(1)
(vi) Increase in pressure will favour backward reaction because np (1) > nr (0).
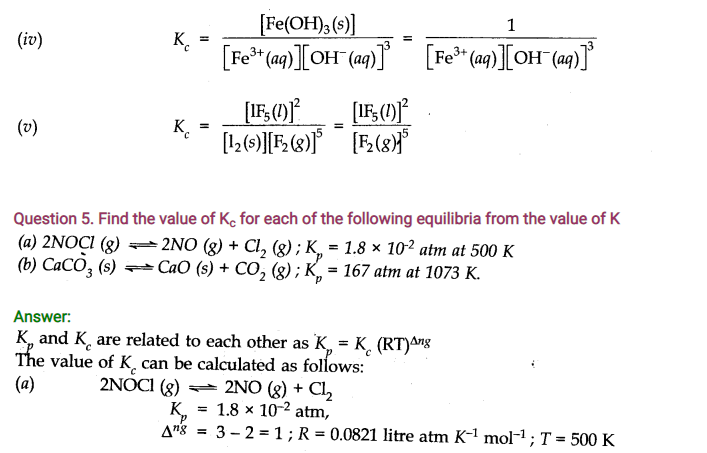
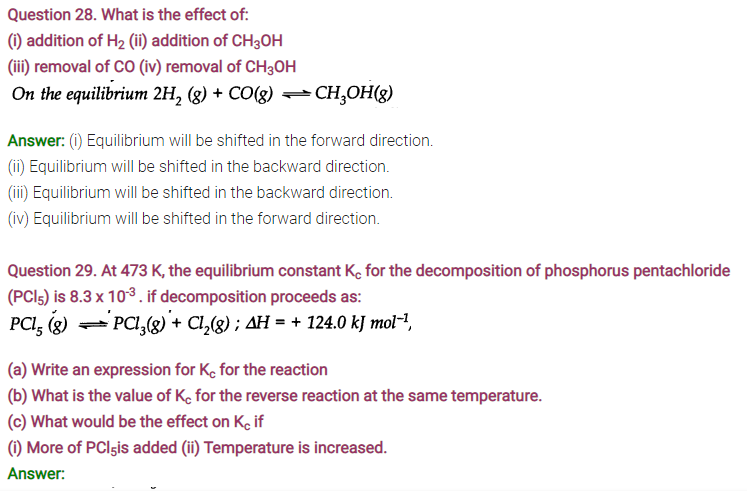
Question 26. The equilibrium constant for the following reaction is 1.6 x 105at 1024 K.
Find the equilibrium pressure of all gases if 10.0 bar of HBr is introduced into a sealed container at 1024 K.


(i) By increasing the pressure, the number of moles per unit volume will increase. In order to decrease the same, the equilibrium gets shifted to the left or in the backward direction. As a result, more of reactants will be formed and the value of Kp will decrease.
(ii) If the temperature is increased, according to Le Chatelier’s principle, the forward reaction will be favoured as it is endothermic. Therefore, the equilibrium gets shifted to the right and the value of Kp will increase.
(iii) The addition of catalyst will not change the equilibrium since it influences both the forward and the backward reactions to the same extent. But it will be attained more quickly.
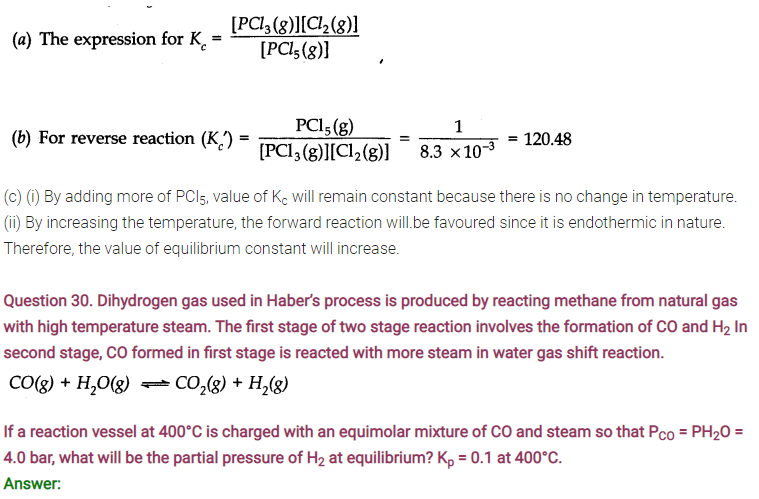

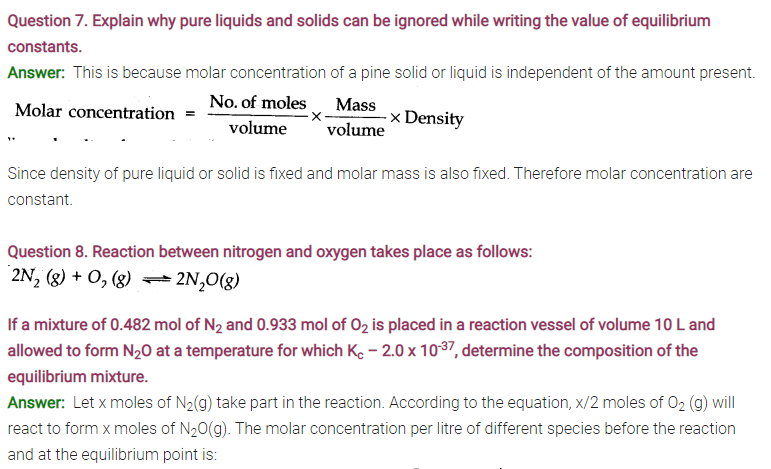
Answer: Following conclusions can be drawn from the values of Kc .
(a) Since the value of Kc is very small, this means that the molar concentration of the products is very small as compared to that of the reactants.
(b) Since the value of Kc is quite large, this means that the molar concentration of the products is very large as compared to that of the reactants.
(c) Since the value of Kc is 1.8, this means that both the products and reactants have appreciable concentration.

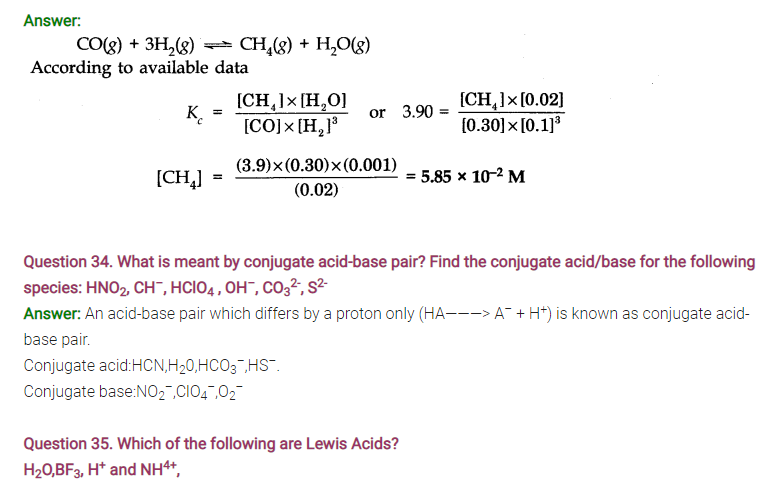
Question 39. Classify the following species into Lewis acids and Lewis bases and show how these can act as Lewis acid/Lewis base?
(a) OH– ions (b) F– (c) H+ (d) BCl3
Answer: (a) OH– ions can demate an electron pair and act as Lewis base.
(b) F– ions can donate an electron pair and act’as Lewis base.
(c) H+ ions can accept an electron pair and act as Lewis acid.
(d) BCl3 can accept an electron pair since Boron atom is electron deficient. It is a Lewis acid.
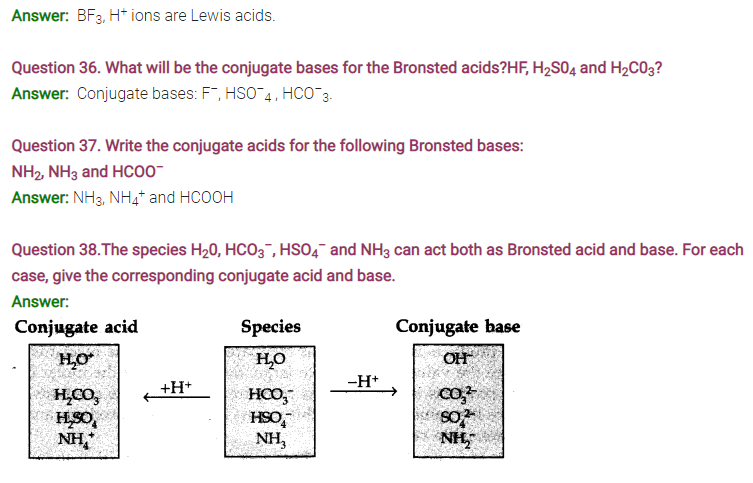
Question 40. The concentration of hydrogen ions in a sample of soft drink is 3.8 x 10-3 M. What is the pH value?
Answer: pH = – log [H+] = – log (3.8 x 10-3) = – log 3.8 + 3 = 3 – 0.5798 = 2.4202 = 2.42
Question 41. The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
Answer: pH = – log [H+] or log [H+] = – pH = – 3.76 = 4.24
.-. [H+] = Antilog 4.24 = 1.738 x 10-4 = 1.74 x 10-4 M
Question 42. The ionization constant of HF, HCOOH and HCN at 298 K are is 6.8 x 10-4 , 1.8 x 10-4 and 4.8 x 10-9 respectively, Calculate the ionization constant of the corresponding conjugate base.
Answer: For F– , Kb =Kw/Ka= 10-14/(6.8 x 10-4) = 1.47 x 10-11 = 1.5 x 10-11 .
For HCOO-, Kb = 10-14/(1.8 x 10-4) = 5.6 x 10-11
For CN–, Kb= 10-14/(4.8 X 10-9) = 2.08 x 10-6

Question 44. The-first ionization constant of H2S is 9.1 x 10-8. Calculate the concentration of HS– ions in its 0.1 M solution and how will this concentration be affected if the solution is 0.1 M in HCl also? If the second dissociation constant of H2S is 1.2 x 10-13, calculate the concentration of S2-under both conditions.
Answer: