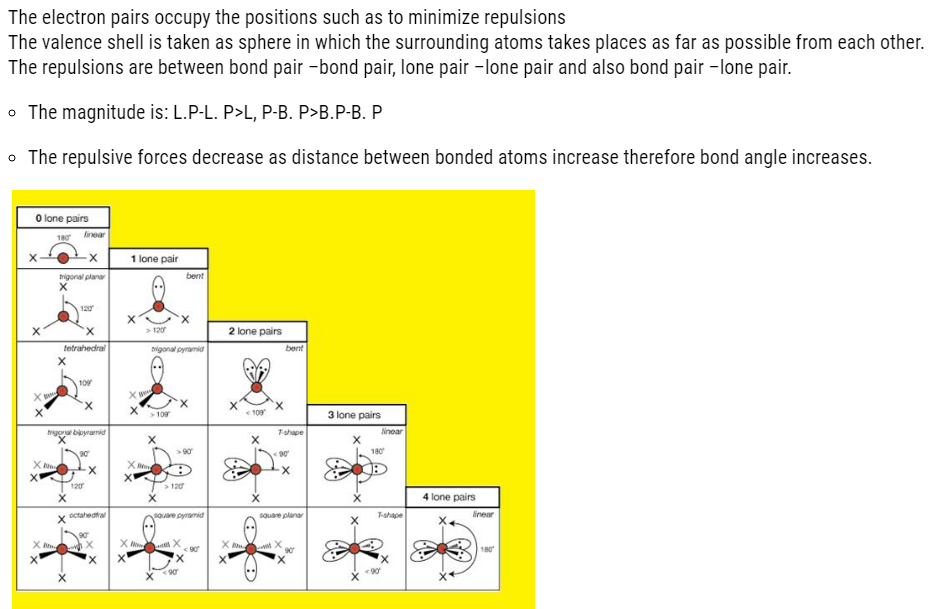
VSEPR THEORY
Geometry of molecule
- It is the relative arrangement of bonded atoms in a molecule.
- The way in which the bonded atoms direct themselves around the central atom is explained on the basis of this theory.
- This theory was given by Sid wick and Favell.
- In this only the valence electrons participate in bond formation.
- The bonded atoms around central metal atom will have repulsions between them
- Therefore, they will arrange themselves in such way that the repulsion is minimum.
In BF3 the arrangement is like it is shown below:


Characteristics
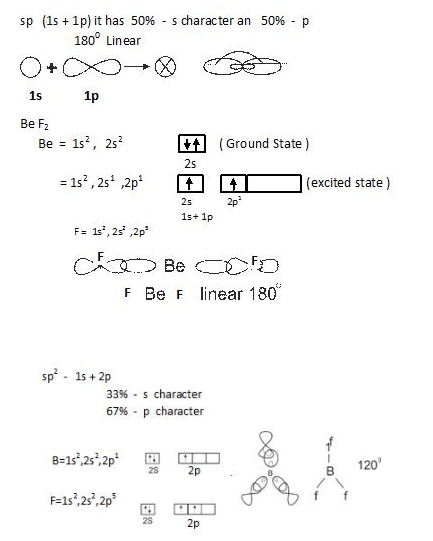
- Number of hybrid orbitals is equal to number of atomic orbitals that combine.
- The hybrid orbitals are always equal in shape and energy.
- The hybrid orbitals are more effective in forming bonds as compared to pure atomic orbitals.
- The hybrid orbitals are directed towards specific directions in space.
- The type of hybridization gives us the shape of molecule
Now there is a question that can all participate in hybridization
Conditions of hybridization
- The only valence orbitals participate.
- The atomic orbitals that participate should have almost same energy.
- Promotion is not always necessary.
- The unpaired as well as fully filled orbitals can also participate.
Types of hybridization
- sp hybridization: In this one s and one p orbital combines as shown below.

Valence bond approach of covalent bond
VSEPR theory does not tell us about bond parameters like directional nature, bond angle, repulsions etc.
To explain we have new theory:
- Valence bond approach
- Molecular orbital theory
- Valence bond theory
Assumptions:
- According to this, the atom retains their identity even after bonding.
- Bond is formed due to interaction of valence electrons only.
- Only the valence electrons lose their identity whereas inner electrons do not participate.
- Stability of bond depends upon amount of energy released
- The molecule has minimum energy at a specific distance called inter-nuclear distance and at that the bond formation takes place.
Overlapping: sigma and pi bond
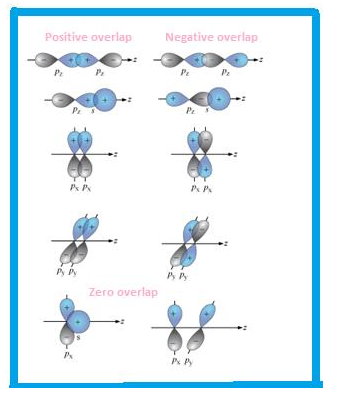
Overlapping can be defined as partial merging of bonded orbitals .More is the overlapping, stronger is the bond formed.
Types of overlapping
- Head to head
- Sidewise
Accordingly, the bond formed is sigma or pi bond.
Sigma bond:
- In this head to head overlapping occurs.
- More is the overlapping region more stable is the bond.
- The bond is stronger.
- This bond can exist independently.
Pi bond:
- It is formed by side wise overlapping of orbitals.
- It is not too strong as in this overlapping region is less.
- It is weaker bond as compared to sigma bond.
- It can’t exist independently, it exist along with sigma bond.
Let’s make Sigma bond :

- Molecular orbital theory
It was developed by F.Hund and R.S Mullikan in 1932.
The features of this theory are:
- The electrons in a molecule are present in various molecular orbital as the electrons of atom are present in different various shells.
- The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
- In molecular orbital electrons are in influence of two or more nuclei.
- The number of molecular orbitals formed is equal to number of atomic orbitals that combine.
- The two orbitals formed due to combination are: Bonding and Anti -bonding.
- The Bonding molecular orbital has lower energy and greater stability than Anti -bonding.
- The electron probability distribution around group of nuclei is given by molecular orbital.
- The molecular orbitals are filled in accordance to Aufbau principle, Pauli’s principle and Hund’s rule.
The linear combination of atomic orbitals to form molecular orbital takes place only if:
- The combining orbitals must have same energy.
- The combining orbitals must have same symmetry.
- The combining orbitals must overlap to maximum extent.
The order to fill molecular orbital is:
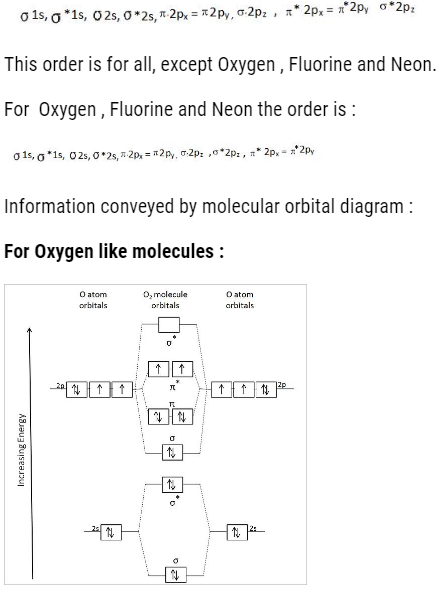
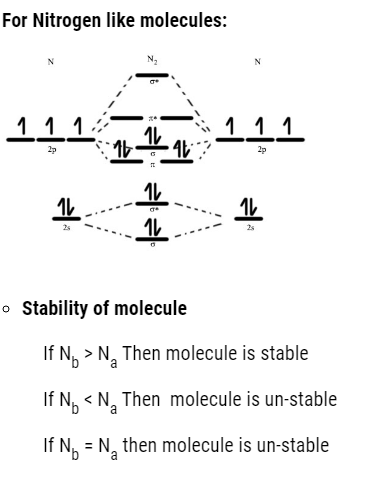
- We can find the bond order : That is
Bond Order 1 (single bond)
Bond Order 2 (double bond)
Bond Order 3 (triple bond)
( c) Tell us about the type of bond :
- Magnetic character :
If unpaired electrons – paramagnetic
If no unpaired electrons – dimagnetic