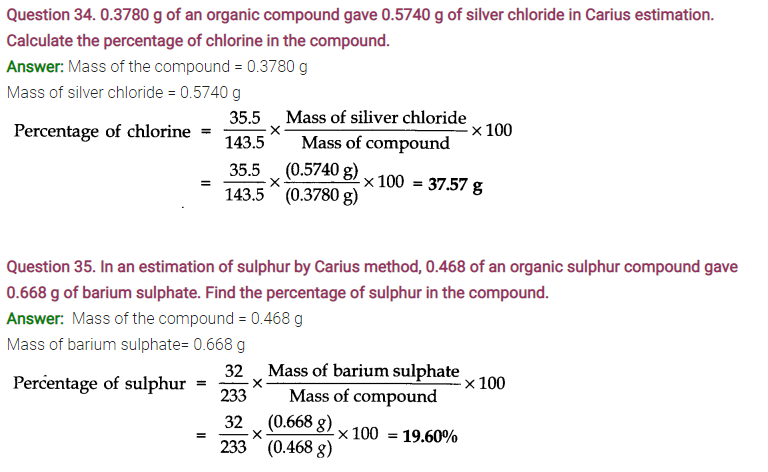
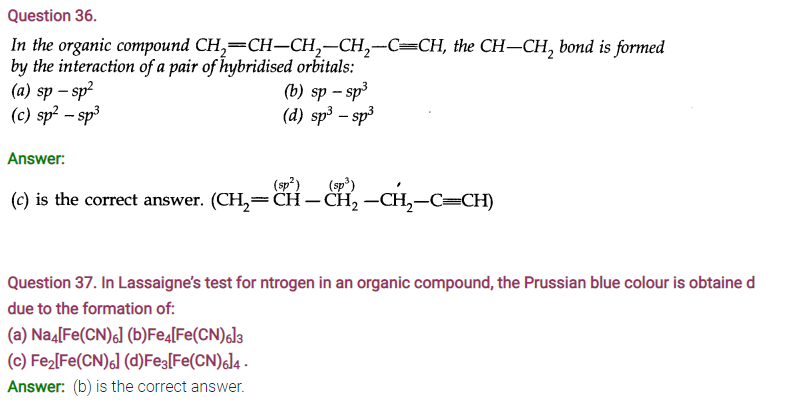
NCERT TEXTBOOK QUESTIONS SOLVED
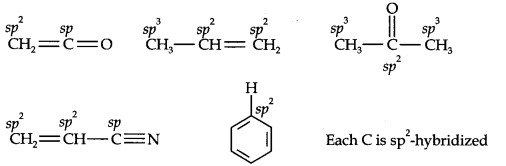
Question 1. What are hybridisation states of each carbon atom in the following compounds? CH2=C=O, CH3CH=CH2, (CH3)2CO, CH2=CHCN, C6H6.
Ans.
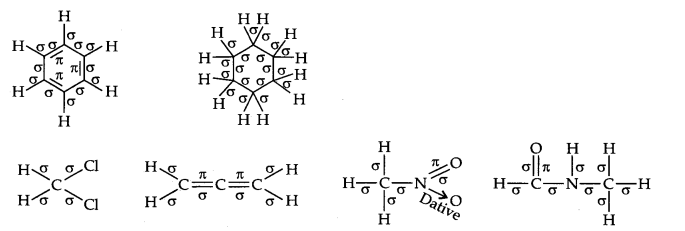
Question 2. Indicate the a- and n-bonds in the following molecules:
C6H6 , C6H12, CH2Cl2, CH=C=CH2, CH3NO2, HCONHCH3
Answer:
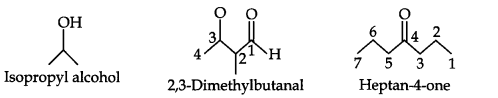
Question 3. Write bond-line formulas for: Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one.
Answer:
Question 4. Give the TUPAC names of the following compounds:
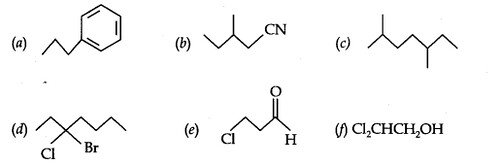
Answer: (a) Propylbenzene (b) 3-Methylpentanenitrite (c) 2, 5-Dimethylheptane
(d) 3-Bromo- 3-chloroheptane (e) 3-Chloropropanal (f) 2, 2-Dichloroethanol
Question 5.Which of the following represents the correct TUPAC name for the compounds concerned?
(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyloctane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- l-ol or But-4-ol-yne.
Answer: (a) 2, 2-Demethylpentane (b)2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7.
(c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-l-ol. Lower locant for the principal functional group, i.e., alcohol.
Question 6. Draw formulas for the first five members of each homologous series beginning with the following compounds,
(a) H—COOH (b) CH3COCH3 (c) H—CH=CH2
Answer: (a) CH3—COOH
CH3CH2—COOH CH3CH2CH2—COOH
CH3CH2CH2CH2—COOH
(b) CH3COCH3
CH3COCH2CH3
CH3COCH2CH2CH3
CH3COCH2CH2CH2CH3
CH3CO(CH3)4CH3
(c) H—CH=CH2
CH3CH=CH2
CH3CH2CH=CH2
CH3CH2CH2CH=CH2
CH3CH2CH2CH2CH=CH2
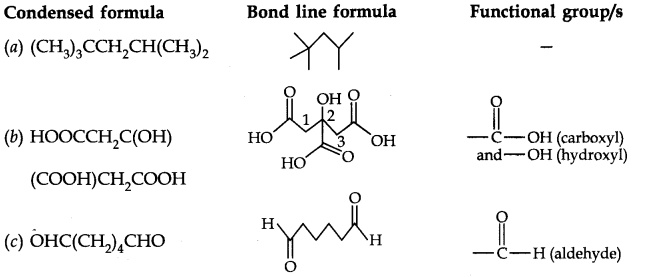
Question 7. Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for: (a) 2, 2, 4-Trimethylpentane (b) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid (c) Hexanedial.
Answer:
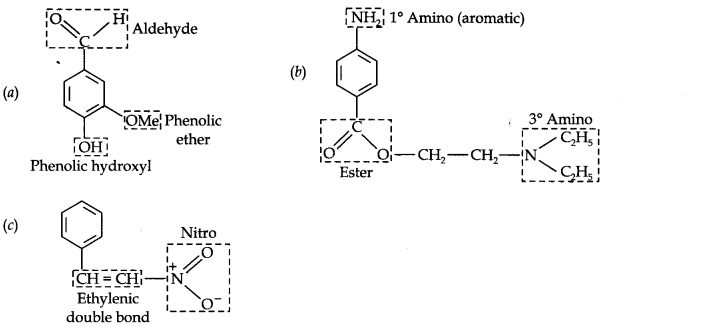
Question 8. Identify the functional groups in the following compounds:

Answer:
Question 9. Which of the two: O2NCH2CH2O– or CH3CH2O– is expected to be more stable and why?
Answer: O2N——<——- CH2——<——- CH2 —<——- O– is more stable than CH3——<——-CH2——<——-O- because NO2 group has -I-effect and hence it tends to disperse the -ve charge on the O-atom. In contrast, CH3CH2 has +I-effect. It, therefore, tends to intensify the -ve charge and hence destabilizes it.
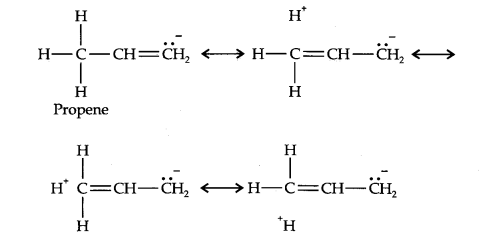
Question 10. Explain why alkyl groups act as electron donors when attached to a π-system.
Answer: Due to hyperconjugation, alkyl groups act as electron donors when attached to a π- system as shown below:
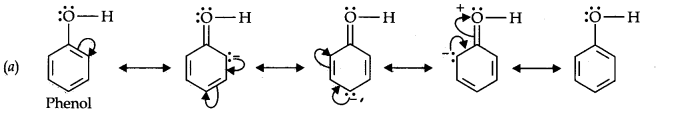
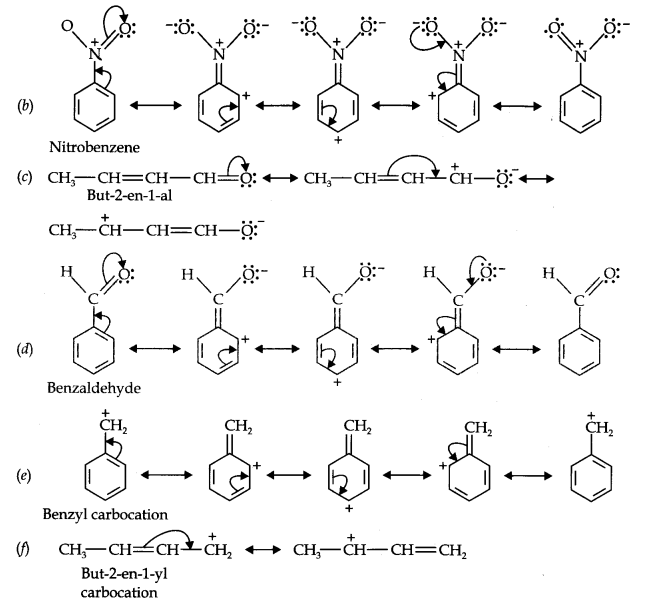
Question 11. Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C6H5OH (b) C6H5N02 (c) CH3CH=CHCHO (d) C6H5—CHO (e) C6H5—CH2 (f) Ch3Ch=ChCh2
Answer:
Question 12. What are electrophiles and nucleophiles? Explain with examples:
Answer: Electrophiles: The name electrophiles means electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules.
Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3
Nucleophiles: The name nucleophiles means ‘nucleus loving’ and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule. They are electron rich they may be negative ions or neutral molecules.
Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
Question 13. Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles
(a) CH3COOH + HO– ———–> CH3COO– + H2O
(b) CH3COCH3 + CN ———–> (CH3)2 C(CN)(OH)
(c) C6H5 + CH3CO ———–> C6H5COCH3
Answer: Nucleophiles: (a) and (b) and Electrophile : (c)
Question 14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– ———–> CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl ———–> (CH3)2CCl—CH3
(c) CH3CH2Br + HO– ———–> CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr ———–> (CH3)2 C Br CH2CH2CH3 + H2O
Answer: (a) Nucleophilic substitution (b) Electrophilic addition
(c)Bimolecular elimination (d) Nucleophilic substitution with rearrangement.
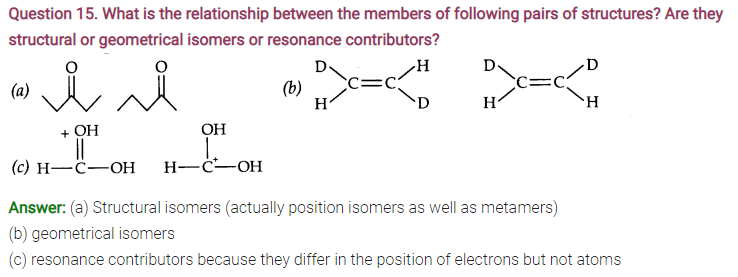

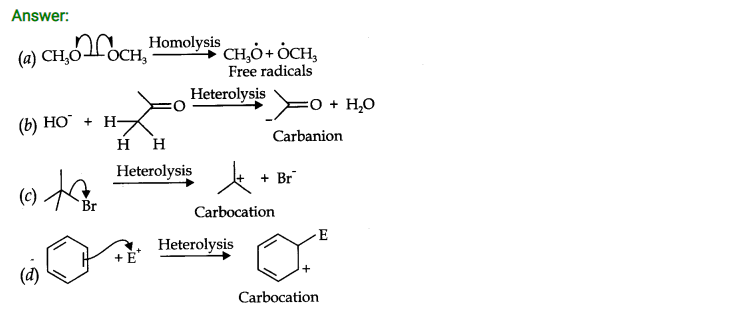
Question 17. Explain the terms inductive and electromeric effects. Which electron displacement effect explain the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
Answer: Inductive Effect: The inductive effect refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another.Atoms or groups which lose electron towards a carbon atom are said to have +1 Effect.
Those atoms or groups which draw electron away from a carbon atom are said to have -I Effect.
Commomexamples of -I effect are:
NO2, F, Cl, Br, I, OH etc.
Examples of +1 effect are (Electron releasing)
(CH3)2C— , (CH3)2CH—, CH3CH2— CH3— etc.
Electromeric effect: The electromeric effect refers to the polarity produced in a multiple bonded compound as it is approached by a reagent.
Question 18. Give a brief description of the principles of the following techniques taking an example in each case: (a) Crystallisation (b) Distillation (c) Chromatography
Answer: (a) Crystallisation: In this process the impure solid is dissolved in the minimum volume of a suitable solvent. The soluble impurities pass into the solution while the insoluble ones left behind. The hot solution is then filtered and allowed to cool undisturbed till crystallisation is complete. The crystals are then separated from the mother liquor by filtraration and dried.
Example: crystallisation of sugar.
(b) Distillation: The operation of distillation is employed for the purification of liquids from non-volatile impurities. The impure liquid is boiled in a flask and the vapours so formed are collected and condensed to give back pure liquid in another vessel. Simple organic liquids such as benzene toluene, xylene etc. can be purified.
(c) Chromatography: Chromatography is based on the principle of selective distribution of the components of a mixture between two phases, a stationary phase and a moving phase. The stationary phase can be a solid or liquid, while the moving phase is a liquid or a gas. When the stationary phase is solid the basis is adsorption and when it is a liquid the basis is partition. Chromatography is generally used for the Reparation of coloured substances such as plant pigments or dyestuffs.
Question 19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Answer: Fractional crystallisation is used for this purpose. A hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out while the more soluble remains in the solution. The crystals are separated from the mother liquor and the mother liquor is again concentrated and the hot solution again allowed to cool when the crystals of the second compound are obtained. These are again filtered and dried.
Question 20. What is the difference between distillation, distillation under reduced pressure and steam distillation?
Answer: Distillation is used in case of volatile liquid mixed with non-volatile impurities.
Distillation under reduced pressure: This method is used to purify such liquids which have very high boiling points and which decompose at or below their boiling points.
Steam distillation is used to purify steam volatile liquids associated with water immiscible impuritites.

Question 22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method.
Answer: (i) Dumas method: The organic compound is heated strongly with excess of CuO ‘ (Cupric Oxide) in an atmosphere of CO2 when free nitrogen, CO2 and H2O are obtained.
(ii)Kjeldahl’s method: A known mass of the organic compound is heated strongly with cone. H2SO4, a little potassium sulphate and a little mercury (a catalyst). As a result of reaction the nitrogen present in the organic compound is converted to ammonium sulphate.


Question 25. Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens ?
Answer: Nitric acid is added to sodium extract so as to decompose
NaCN + HNO3 ——-> NaNO3 + HCN
Na2S + 2HNO3 ——> 2NaNO3 + H2S
Question 26. Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Answer: Organic compound is fused with sodium metal so as to convert organic compounds into NaCN, Na2S, NaX and Na3PO4. Since these are ionic compounds and become more reactive and thus can be easily tested by suitable reagents.
Question 27. Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Answer: Sublimation.Because camphor can sublime whereas CaSO4 does not.
Question 28. Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation ?
Answer: It is because in steam distillation the sum of vapour pressure of organic compound and steam should be equal to atmospheric pressure.
Question 29.Will CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer.
Answer: No. CCl4 is a completely non-polar covalent compound whereas AgNO3 is ionic in nature. Therefore they are not expected to react and thus a white ppt. of silver chloride will not be formed.
Question 30. Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound?
Answer: CO2 is acidic in nature and therefore, it reacts with the strong base KOH to form K2CO3.
2KOH + CO2 ——–> K2CO3+ H2O.

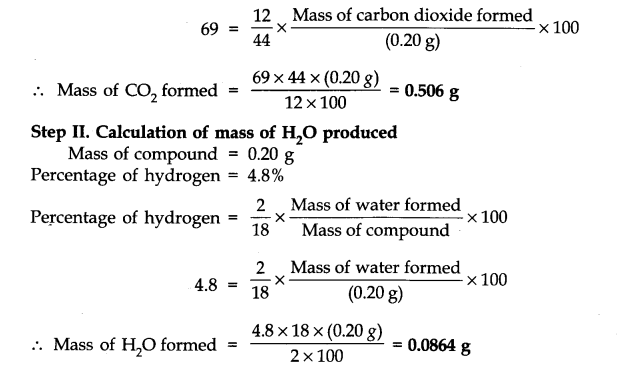
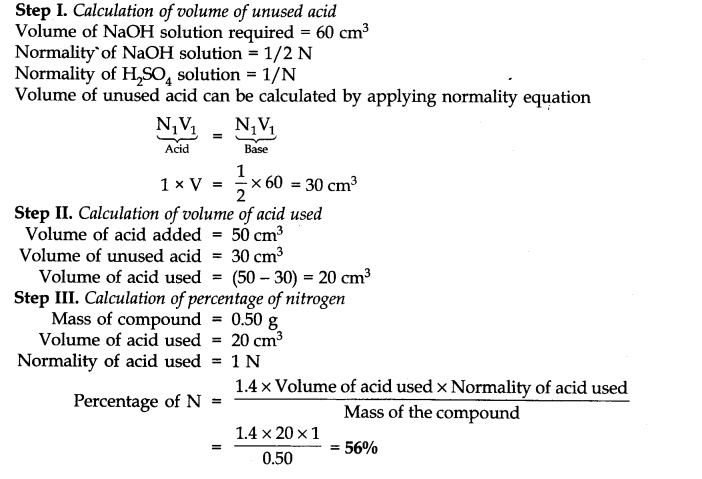
Question 33. 0.50 g of an organic compound was Kjeldahlished. The ammonia evolved was passed in 50 cm3 of IN H2SO4. The residual acid required 60 cm3 of N/2 NaOH solution. Calculate the percentage of nitrogen in the compound.
Answer: