About Lesson
Bond parameters
They are the measurable properties of a bond and these are associated only with covalent bond.
- Bond length: It is the average distance between the centre of two bonded atoms .
Factors on which bond length depends:
- Size of the atoms: Bigger the size of the atom more is the bond length.
- Multiplicity of bond: If there is a single bond, bond length is longer as compared to bond length in double or triple bond.
- That is the reason bond length of alkanes is more than alkenes which is further more than alkynes.
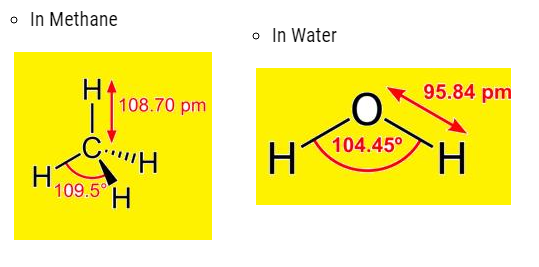
- Bond angle: It is an average distance between the orbital of the atoms surrounding the central atom.
For example

- Bond order: It is the number of bonds present between two atoms.
For example: In H2 (bond order is 1 )
In oxygen molecule (bond order is 2)
In nitrogen molecule bond order is 3)
- In Iso-electronic species: The bond order is same.
Example: In Fluorine and O22- the electrons are 18 and bond order is same that is 1.

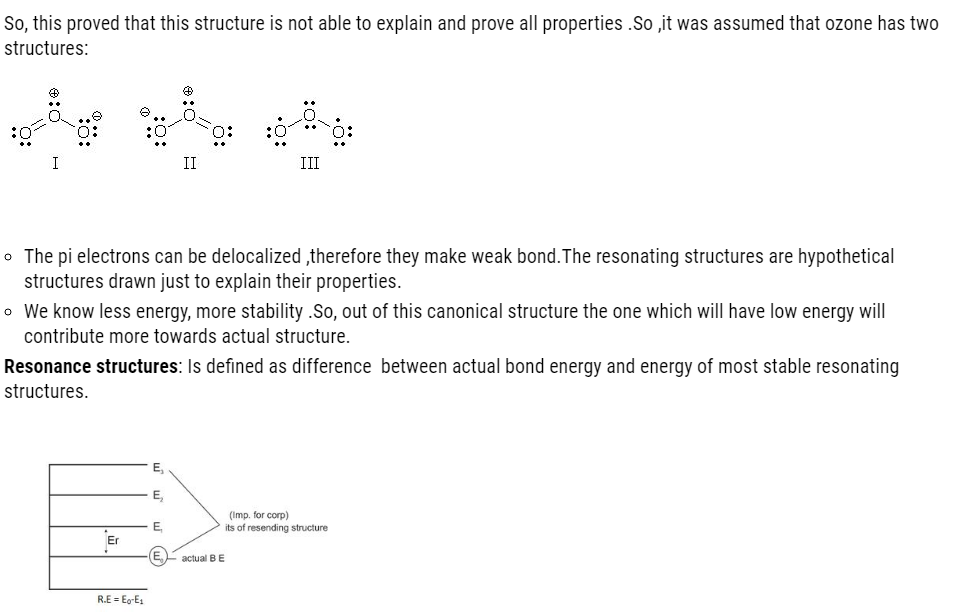
- Resonance averages the bond characteristics of a molecule as a whole.
- Resonance stabilizes the molecule because energy of the resonance hybrid is less than the energy of any canonical forms.
Characteristics
- It has no real existence.
- Resonance has the same bond length.
- A resonance hybrid has the lowest energy.
- The greater the resonance and resonance energy the more stable is the structure.
- Resonance is a theoretical concept with no experimental verification.,