Physical Properties of alkanes
- Polarity of alkanes: Alkanes are non-polar in nature because of same electro negatives of carbon atoms.
- Existence: The force of attraction present in them is Vander wall forces due to which the lower members i.e. C1 to C4 exist as gases, C5 – C7 as liquid and higher ones are solid.
- Boiling point: – Because of non-polar character they have low boiling point. The boiling points increase with increase in the number of carbon atoms due to increase in Vander wall forces of attraction. However, with increase in branching the surface area decreases and hence, Vander wall force decreases and hence the boiling point decreases.
For example: CH3 CH2CH2 CH3 butane has more boiling point then 2 methyl propane
(d)Melting Point: – It not only depends on mass but also on structure of hydrocarbon.
- So, alkane with odd number of carbon atom has low melting point
- Alkane with even number of carbon atoms have high melting point
- Because they have symmetrical structure, so they fit better in lattice due to this their
Melting point is high.
For example: Pentane has low melting point than hexane because hexane has symmetrical structure.
(e)Solubility: We know Hydrocarbons are non-polar and we also know like dissolve like
So they are not soluble in H2O but soluble in organic solvents like ether CCl4,
(f)Density: It is lighter than water. It increases with increase number of C atoms.
Chemical properties of alkanes
We know that on saturated Hydrocarbons are quite less reactive and undergo reaction with difficulty therefore they are called paraffin’s.
The reactions shown by them are given below:
- 1st Substitution reaction: In this one or more Hydrogen’s are replaced by other atoms.
For example
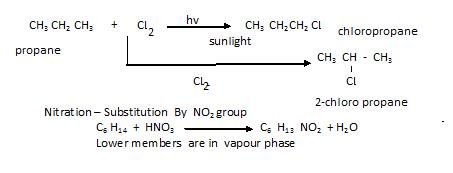
- Halogenations– In it the Substitution By halogen X (Cl, F, Br, I) occur
Order of reactivity: F> Cl > Br > I
Out of them chlorine shows this reaction to an efficient level.
Chlorination of methane: This reaction occurs in normal light
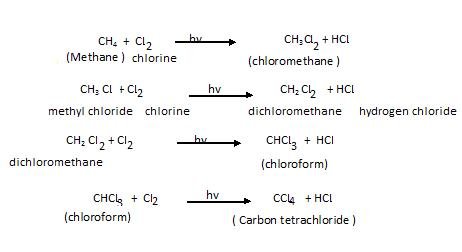
It involves Free radical mechanism as shown below:

If we take comparatively bigger alkane then we get isomers as shown below in example:
- 2nd Oxidation Reaction: it is the Reaction with oxygen
This reaction may be may be uncontrolled or controlled oxidation. If it is combustion reaction than the products are always CO2, H2O, heat and light.
- If it is Complete combustion : then also the products are CO2, H2O, heat and light
CH4 + O2 à CO2 + H2O + Heat
(Methane) Oxygen Gas Carbon dioxide
- Incomplete combustion or oxidation :
CH4 + O2 à CO + H2O + Heat
(Methane) Oxygen Gas Carbon monoxide
This CO makes the surface block and called Carbon black or soot.
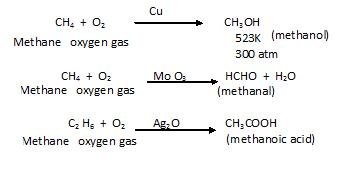
- Controlled oxidation – occur in presence of catalyst and on the basis of catalyst the products are formed. Like in presence of Copper alcohol is formed, in presence of molybdenum oxide – aldehyde is formed and in presence of silver oxide –carboxylic acid is formed.
- 3rd Isomerisation
If we subject alkanes to (anhydrous) Aluminium chloride then isomer of alkane is formed.
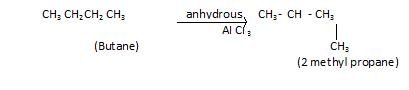
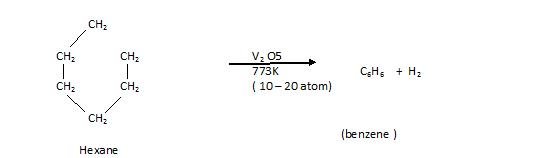
4th Aromatization Reaction: In this an Aromatic compounds are found are called (Cyclization)
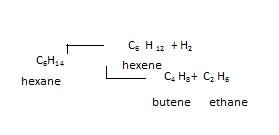
5th Thermal decomposition or pyrolysis: In this breakdown of bigger alkane into lower alkanes occur and is called as thermal cracking.