1 Marks Questions
1.What is chemistry?
Ans: Chemistry is the branch of science that studies the composition, properties and interaction of matter.
2.How has chemistry contributed towards nation’s development?
Ans: chemical principles are important in diverse areas such as weather patterns, functioning of brain, operation of a computer, chemical industries, manufacturing , fertilizers, alkalis, acids, salts, dyes, polymers, drugs, soaps, detergents, metals, alloys, contribute in a big way to national economy.
3.Differentiate solids, liquids & gases in terms of volume & shapes.
Ans:
|
Property |
Solids |
Liquids |
Gases |
|
1. Volume |
Definite |
Definite |
Not definite |
|
2. Shape |
Fixed |
Not fixed, take the shape of container, |
Not fixed, takes the shape of the container |
4.Name the different methods that can be used for separation of components of a mixture. .
Ans:The components of a mixture can be separated by physical methods like handpicking, filtrations, crystallization, distillation etc.
5.Classify following as pure substances and mixtures – Air, glucose, gold, odium and milk.
Ans:
| Pure Substances | Mixtures |
| Glucose | Air |
| Gold | Milk |
| Sodium |
6.What is the difference between molecules and compounds? Give examples of each.
Ans: Molecules consist of different atoms or same atoms. e.g. molecule of hydrogen contains two atoms of hydrogen where as molecule of water contain two atoms of hydrogen and one of oxygen.
Compound is formed when two or more than two different atoms combine in fire propo e.g. water –rtion carbondioxide, sugar etc.
7. How can we separate the components of a compound?
Ans:The constituents of a compound can not be separated by physical methods. They can only be separate by chemical methods.
8.How are physical properties different from chemical properties?
Ans: Physical properties are those properties which can be measured or observed without changing the identity or the composition of the substance whereas the measurement of chemical properties require a chemical change to occur e.g. colour, odour etc are physical properties and combustion, basicity etc are chemical properties.
9.What are the two different system of measurement?
Ans:The different system of measurement are English system and the metric system.
10.What is the SI unit of density?
Ans: The SI Unit of density is Kg m-3 or kg/m3
11.What are the reference points in thermometer with Celsius scale?
Ans:The thermometers with Celsius scale are calibrated form 0o to 100o where there two temperatures are the freezing and boiling of water.
12.What is the SI unit of volume? What is the other common unit which in not
an SI unit of volume.
Ans: The SI unit of volume is m3 whereas litre (L) is the common unit which is not an SI unit.
13.What is the difference between precision and accuracy?
Ans:Precision means the closeness of various measurements for the same quantity. Accuracy is the agreement of a particular value to the true value of the result.
14.What do you understand by significant figures?
Ans:Significant figures are meaningful digits which are known with certainty. The uncertainty in experimental or the calculated value is indicated by mentioning the number of significant figures.
15.State law of definite proportions.
Ans: Law of definite proportions states that a given compound always contains exactly the same proportion of elements by weight.
16.State Avogadro’s law.
Ans:According to Avogadro’s law, equal volumes of gases at the same temperature and pressure should contain equal number of molecules.
17.Define one atomic mass unit (amu).
Ans: One atomic mass unit (amu) is defined as a mass exactly equal to one – twelfth the mass of one carbon – 12 atom.
18.What is formula mass?
Ans: When a substance does not contain discrete molecules as their constituent units and have a three dimensional structure, formula mass is used to calculate molecular mass which is sum of all the atomic masses of atom present in the formula.
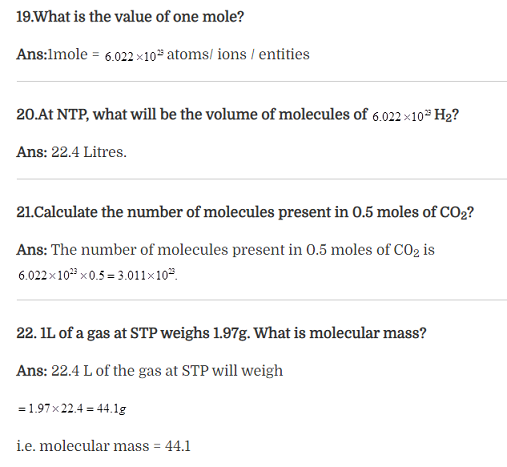
23.What is stoichiometry?
Ans: Stoichimetry deals with the calculations of masses of reactants and products involved in a chemical reactions.
24. The substance which gets used up in any reaction is called ————–
Ans: The substance that gets used up in any reaction is called limiting reagent.
25.What is 1molal solution?
Ans: one molal solution is solution in which one mole of solute is present in 1000g of solvent.
2 Marks Questions
How can we say that sugar is solid and water is liquid?
Ans: Sugar has close packing of constituent particles, have its own volume and shape therefore, it can be said to be solid whereas in water the constituent particles are not as closely packed as in solid. It has definite volume but not definite shape. Therefore it is a liquid.
Classify following substances as element, compounds and mixtures – water,
tea, silver, steel, carbondioxide and platinum
Ans:
| Compounds | Elements | Mixtures |
| Water | Silver | Tea |
| Carbondioxide | Platinum | Steel |
Write seven fundamental quantities & their units.
Ans:
| Physical Quantity | SI unit |
| 1. Length (l) | Metre (m) |
| 2. Mass (m) | Kilogram (kg) |
| 3. Time (t) | Second (s) |
| 4. Electric Current (I) | Ampere (A) |
| 5. Thermodynamic Temperature (T) | Kelvin (K) |
| 6. Amount of substance (n) | Mole (mol) |
| 7. Luminous Intensity (I) | Candela (Cd) |
What is the difference between mass & weight? How is mass measured in laboratory?
Ans: Mass of a substance is the amount of matter present in it while weight is the force exerted by gravity on an object the mass of a substance is determined with the help of an analytical balance in laboratory.
What does the following prefixes stand for –
(a) pico
(b) nano
(c) centi
(d) deci
Ans: Pico = 10-12
nano = 10-9
centi = 10-2
deci = 10-1
Write Postulates of Dalton’s atomic theory.
Ans. Postulates of Dalton’s atomic theory –
1. Matter consists of indivisible atoms.
2. All the atoms of a given element have identical properties including atomic mass. Atoms of different element differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reaction involves reorganization of atoms. These are neither created nor destroyed
Give one example each of a molecule in which empirical formula
and molecular formula are (i) same (ii) Different.
Ans:(i) Same molecular formula and empirical formula. Carbon dioxide, both is CO2.
(ii) When molecular formula and empirical formula are different –
Hydrogen peroxide: molecular formula is H2O2 and empirical formula is HO
How many significant figures are present in
(a) 4.01  102
102
(b) 8.256
(c) 100
Ans:(a) 4.01  102 – Three
102 – Three
(b) 8.256 – Four
(c) 100 – One