I. Very Short Answer Type Questions
Question 1. How will you separate a mixture of two organic compounds which have different solubilities in the same solvent?
Answer: By fractional crystallisation.
Question 2. An organic liquid decomposes below its boiling point. How will you purify it?
Answer: By distillation under reduced pressure.
Question 3. Suggest a suitable technique for separating naphthalene from kerosene oil present in a mixture.
Answer: Simple distillation.
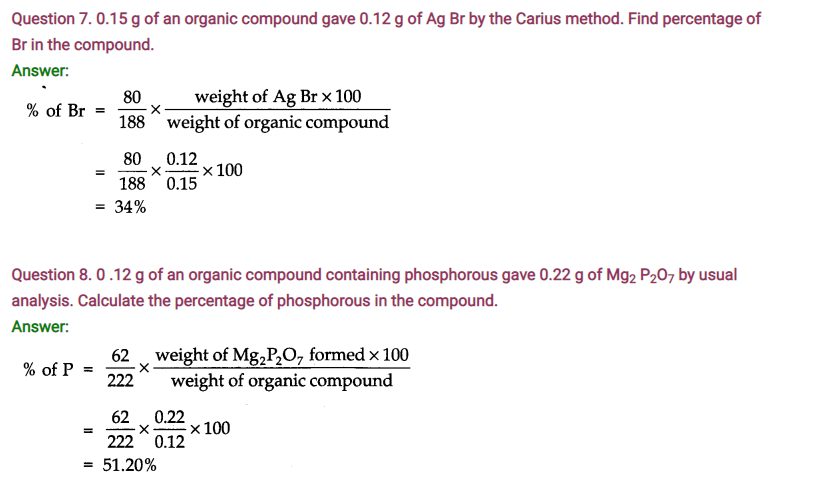
Question 4. Arrange the following in increasing order of C—C bond length: C2H & C2H4, C2H2.
Answer: C2H2 (120 pm) < C2H4 (134 pm) < C2H6 (154 pm)
Question 5. Name the process used to separate sugar and salt.
Answer: Fractional crystallisation using ethanol as a solvent.
Question 6. Which gas is liberated in Kjeldhal’s method?
Answer: Ammonia gas (NH3)
Question 7. What is Lassaigne’s extract?
Answer: When organic compound is fused with sodium metal and then extracted by water, it is called Lassaigne’s extract.
Question 8. What type of solids are separated by fractional crystallisation?
Answer: Those solids which are soluble in the same splvent but to a different extent i.e., differ in their solubility.
Question 9. Name a suitable adsorbent used in the process of column chromatography.
Answer: Al2O3 (alumina)
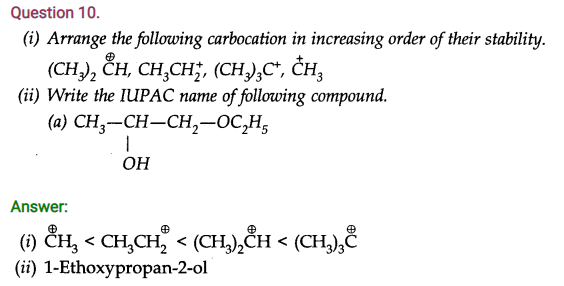
Question 10. Name three types of chromatography.
Answer: Column chromatography, paper chromatography and thin layer chromatography.
Question 11. Which method is used to extract a compound in aqueous solution?
Answer: Differential extraction.
Question 12. In Carius method, sulphur is estimated by precipitating it as which compound?
Answer: BaSO4.
Question 13. Which elements are estimated by Liebig’s Method?
Answer: Carbon and hydrogen.
Question 14. Which type of compounds are purified by steam distillation?
Answer: Steam volatile and insoluble in water.
Question 15. Complete the following:(CH3COO)2 Pb + Na2S ———->
Answer: (CH3COO)2 Pb + Na2S ———->PbS + 2CH3COONa
Question 16. How will you separate a mixture of Iodine and sodium chloride!
Answer: Sublimation.
Question 17. Why is an organic compound fused with sodium in Lassaigne’s test?
Answer: It is because sodium is highly reactive and it reacts with elements to form ionic compounds.
Question 18. Write the name of element which is confirmed on adding Na2[Fe(CN)5NO] in sodium extract solution due to appearance of violet colouration.
Answer: Sulphur.

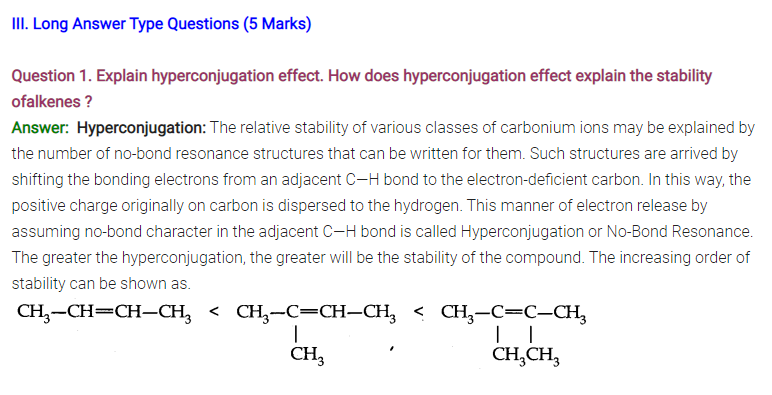
Question 2. (a) What do you understand by Homolytic fission?
(b) What are carbanions? Give an example.
Answer: (a) Homolytic fission is breaking of a bond in such a manner that each atom takes one electron each to form free radicals.
A——-B ———-> A + B
(b) Organic ions which contain a negatively charged carbon atom are called carbanions. e.g., CH3–is carbanion.
Question 3. How will you detect the presence of nitrogen and sulphur in Lassaigne’s extract?
Answer: If freshly prepared FeSO4 and then dil. H2SO4 is added to Lassaigne’s extract, a blue green colouration confirms the nitrogen.
Question 4. Give equation for the following:
(i) Electrophilic Substitution
(ii) Nucleophilic Substitution
Answer:



Question 9. (a) Which is more suitable method for the purification of a compound in liquid state which decomposes at or below its boiling point?
(b) How will you separate a mixture of ammonium chloride and common salt?
Answer: (a) Distillation under reduced pressure or vacuum distillation (b) Sublimation.
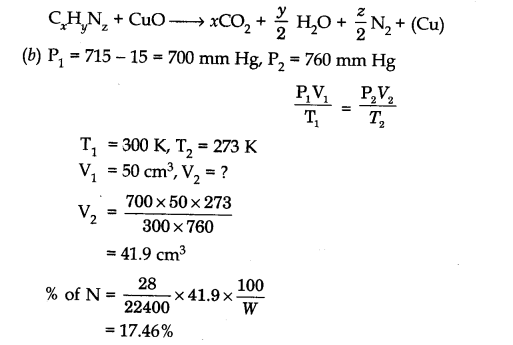
Question 2. (a) What is the basic principle involved in the estimation of nitrogen by Dumas method.
(b) In a Dumas nitrogen estimation method, 0.30 g of an organic compound gave 50 cm3 of N2 collected at 300 K and 715 mm Hg pressure. Calculate the percentage composition of nitrogen in the compound. (Vapour pressure of water at 300 K is 15 mm Hg)
Answer: (a) This method is based upon the fact that nitrogenous compound is heated with
copper oxide in an atmosphere of carbon dioxide yield free nitrogen.
Question 3. (a) What is Lassaigne’s extract? Will NaCN give a positive Lassaigne’s test for nitrogen?
(b) Which colour will appear in the Lassaigne’s test if the compound contains both nitrogen and sulphur.
(c) Why is Lassaigne’s extract prepared in distilled water? Can we detect oxygen in a compound by Lassaigne’s test?
Answer: (a) When organic compound is fused with sodium metal and then extracted by water, it is called Lassaigne’s extract. Yes.
(b) Blood red colour.
(c) Lassaigne’s extract is prepared in distilled water since tap water contains CL ions. No, oxygen cannot be detected by Lassaigne’s test.