NCERT Solutions for Class 11 Chemistry Chapter 6 Thermodynamics
NCERT Class 11 Chemistry Textbook QUESTIONS SOLVED
Question 1. Choose the correct answer:
A thermodynamic state junction is a quantity
(i) used to determine heat changes
(ii) whose value is independent of path
(iii) used to determine pressure volume work
(iv) whose value depends on temperature only.
Answer: (ii) whose value is independent of path
Question 2. For the process to occur under adiabatic conditions, the correct condition is:
(i) ∆T= 0 (ii) ∆p = 0
(iii) q = 0 (iv) w = 0
Ans. (iii) q = 0
Question 6. A reaction, A + B—>C + D + q is found to have a positive entropy change. The reaction will be
(i) possible at high temperature (ii) possible only at low temperature
(iii) not possible at any temperature (iv) possible at any temperature
Answer: (iv) possible at any temperature
Question 7. In a process, 701 ] of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy for the process?
Answer: Heat absorbed by the system, q = 701 J Work done by the system = – 394 J Change in internal energy (∆U) = q + w = 701 – 394 = 307 J.
Question 8. The reaction of cyanamide,NH2CN(s) with dioxygen was carried out in a bomb calorimeter and ∆U was found to be -742,7 KJ-1 mol-1 at 298 K. Calculate the enthalpy change for the reaction at 298 K.NH2CN (S) + 3/202(g) —–>N2(g) + CO2(g) + H20(Z)
Answer: ∆U = – 742.7 KJ-1 mol-1 ; ∆ng = 2 – 3/2 = + 1/2 mol.
R = 8.314 x 10-3KJ-1 mol-1 ; T = 298 K
According to the relation,∆H = ∆U+∆ngRT
∆H = (- 742.7 kj) + (1/2 mol) x (8.314 x10-3 KJ-1 mol-1 ) x (298 K)
= – 742.7 kj + 1.239 kj = – 741.5 kj.
Question 9. Calculate the number of kj of heat necessary to raise the temperature of 60 g of aluminium from 35°C to 55°C. Molar heat capacity of Al is 24 J mol-1 K-1.
Answer: No. of moles of Al (m) = (60g)/(27 g mol-1) = 2.22 mol
Molar heat capacity (C) = 24 J mol-1 K-1.
Rise in temperature (∆T) = 55 – 35 = 20°C = 20 K
Heat evolved (q) = C x m x T = (24 J mol-1 K-1) x (2.22 mol) x (20 K)
= 1065.6 J = 1.067 kj
Question 11. Enthalpy of combustion of carbon to carbon dioxide is – 393.5 J mol-1 .Calculate the heat released upon formation of 35.2 g of C02 from carbon and oxygen gas.
Answer: The combustion equation is:
C(s) + 02 (g) —–> C02(g); AcH = – 393.5 KJ mol-1
Heat released in the formation of 44g of C02 = 393.5 kj
Heat released in the formation of 35.2 g of C02=(393.5 KJ) x (35.2g)/(44g) = 314.8 kj
Question 12. Calculate the enthalpy of the reaction:
N204(g) + 3CO(g) ———->N20(g) + 3CO2(g)
Given that;∆fH–CO(g) = – 110 kj mot-1; ∆fHC02(g) = – 393 kj mol-1
∆fHN20(g) = 81 kj mot-1; ∆fN2O4(g) = 9.7 kj mol-1
Answer: Enthalpy of reaction (∆r,H) = [81 + 3 (- 393)] – [9.7 + 3 (- 110)]
= [81 – 1179] – [9.7 – 330] = – 778 kj mol-1
Question 13. Given : N2(g) + 3H2(g) ————> 2NH3(g); ∆r H– = -92.4 kj mot-1 What is the standard enthalpy of formation of NH3 gas?
Answer: ∆H– NH3 (g) = – (92.4)/2 = – 46.2 kj mol-1
Question 14. Calculate the standard enthalpy of formation of CH3OH. from the following data:
(i) CH3OH(l) + 3/2 02 (g) ———-> CO2 (g) + 2H20 (l); ∆rH– = – 726kj mol-1
(ii) C(s) + 02(g) —————>C02 (g); ∆cH– = -393 kj mol-1
(iii) H2(g) + 1/202(g) —————->H20 (l); ∆fH– = -286 kj mol-1
Answer: The equation we aim at;
C(s) + 2H2(g) + l/202(g) ———> CH3OH (l);∆fH– = ±? … (iv)
Multiply eqn. (iii) by 2 and add to eqn. (ii)
C(s) + 2H2(g) + 202(g) ————->C02(g) + 2H20(Z)
∆H = – (393 + 522) = – 965 kj moH Subtract eqn. (iv) from eqn. (i)
CH3OH(Z) + 3/202(g) ————> C02(y) + 2H20(Z); ∆H = – 726 kj mol-1
Subtract: C(s) + 2H2(y) + l/202(g) ———-> CH3OH(Z); ∆fHe = – 239 kj mol-1
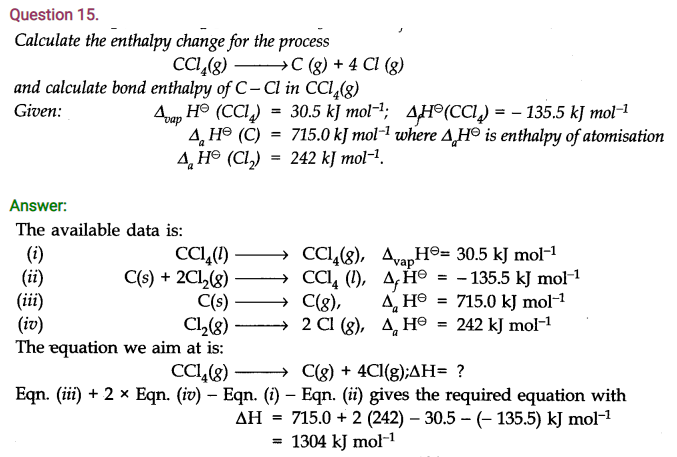

Question 18. For the reaction; 2Cl(g) ———-> Cl2(g); what will be the signs of ∆H and ∆S?
Answer: ∆H : negative (- ve) because energy is released in bond formation
∆S : negative (- ve) because entropy decreases when atoms combine to form molecules.