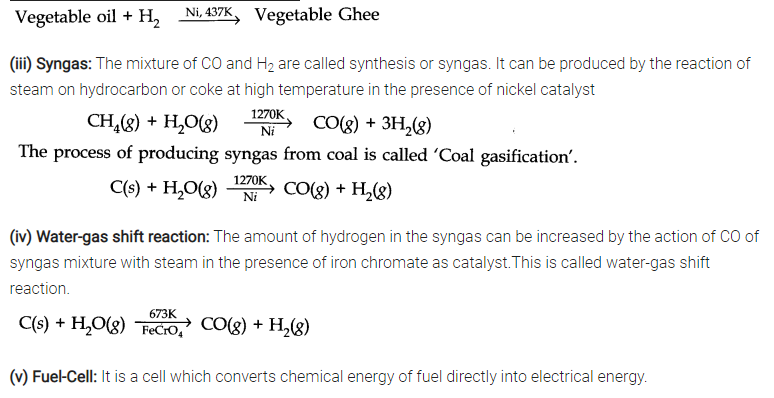NCERT Class 11 Chemitry Textbook Questions Solved
Question 1. Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer: Hydrogen has been placed at the top of the alkali metal in group, but it is not a member of the group.
Its position is not justified properly because of its electronic configuration as (1s1). It can be placed with alkali metals because it also has similar configuration (ns1) as alkali metals.
However, it can also be placed along with halogen in group 17 since just like halogen it can acquire inert gas configuration by accepting one electron.
Question 2. Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes?
Answer:

Question 3. Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer: In diatomic form, the K-shell of hydrogen is complete (1s2) and so it is quite stable.
Question 4. How can the production of dihydrogen obtained from ‘Coal gasification’ be increased?
Answer: The production of dihydrogen in coal gasification can be increased by reacting CO(y) present in syngas with steam in the presence of iron chromate catalysts.

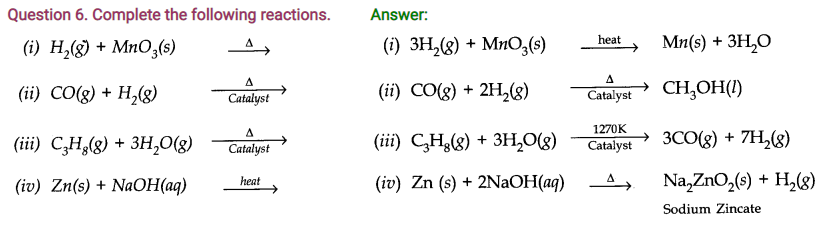
Question 7. Discuss the consequences of high enthalpy of H-H bond, in terms of chemical reactivity of dihydrogen.
Answer: This is due to its small atomic size and also small bond length (74 pm) of H-H bond.
Question 8. What do you understand by (i) Electron-deficient (ii) Electron-precise (iii) Electron-rich compounds of hydrogen? Provide justification with suitable examples.
Answer: (i) Electron deficient hydrides: Compounds in which central atom has incomplete octet, are called electron deficient hydrides. For example, BeH2, BH3 are electron deficient hydrides.
(ii) Electron precise hydrides: Those compounds in which exact number of electrons are present in central atom or the central atom contains complete octet are called precise hydrides e.g., CH4, SiH4, GeH4 etc. are precise hydrides.
(iii) Electron rich hydrides: Those compounds in which central atom has one or more lone pair of excess electrons are called electron rich hydrides, e.g.,NH3, H2O.
Question 9. What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reaction?
Answer: It is expected to be a Lewis acid. They are likely to accept electrons to become stable. They can form coordinate bond with electron rich compound.

Question 12. How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer: In metallic hydrides, hydrogen is adsorbed as H-atoms. Due to the adsorption of H atoms the metal Lattice expands and become unstable. Thus, when metallic hydride is heated, it decomposes to form hydrogen and finely divided metal. The hydrogen evolved can be used as fuel.
Question 13. How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes ? Explain.
Answer: When hydrogen is burnt in oxygen the reaction is highly exothermic, it produces very high temperature nearly 4000°C which is used for cutting and welding purposes.
Question 14. Among NH3 H2O and HE, which would you expect to have highest magnitude of hydrogen bonding and why?
Answer: HF is expected to have highest magnitude of hydrogen bonding since, ‘F’ is most electronegative. Therefore, HF is the most polar.
Question 15. Saline hydrides are known to react with water violently producing fire. Can C02, a well known fire extinguisher, be used in this case? Explain.
Answer: No. Because if saline hydrides react with water the reaction will be highly exothermic thus the hydrogen evolved in this case can catch fire. C02 cannot be used as fire extinguisher because C02 will get absorbed in alkali metal hydroxides.
Question 16. Arrange the following:
(i) CaH2, BeH2 and TiH2 in order of increasing electrical conductance.
(ii) LiH, NaH and CsH in order of increasing ionic character.
(iii) H-H, D—D and F—F in order of increasing bond dissociation enthalpy.
(iv) NaH, MgH2 and H2O in order of increasing reducing property.
Answer: (i) BeH2< TiH2 < CaH2
(ii) LiH < NaH < CsH
(iii) F—F < H—H < D—D
(iv) H2O < MgH2 < NaH

Question 20. Complete the following chemical reactions.
(i) PbS(s) + H2O2 (aq) ————->
(ii) MnO4– (aq) + H2O2 (aq) ————->
(iii) CaO(s) + H2O(g) ————->
(iv) AlCl3(g) + H2O(l)————->
(v) Ca3N2(S) + H2O(l) ————->
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Answer: (i) PbS(s) +4H2O2(aq) ————-> PbSO4(s) + 4H2O(l)
(ii) 2MnO4– (aq) +H2O2(aq) + 6H+(aq) ————-> 2Mn (aq) + 8H2O(l) + 5O2(g)
(iii) CaO(s) + H2O(g) ————->Ca(OH)2(aq)
(iv) AlCl3(aq) + 3H2O(l) ————-> Al(OH)3(S) + 3HCl (aq)
(v) Ca3N2(s) + H2O(l) ————->3Ca(OH)2(aq) + 2NH3(aq)
(a) Hydrolysis reactions, (iii) (iv) and (v)
(b) Redox reactions (i) and (ii)

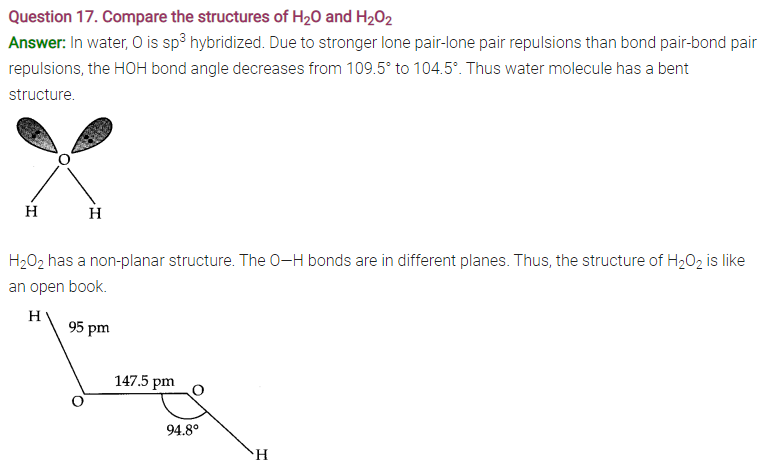
Answer: Ice crystallizes in the normal hexagonal form. However, at very low temperatures it condenses in cubic form. In the normal hexagonal ice each oxygen atom is tetrahedrally surrounded by four other hydrogen atoms.
Question 22. What causes the temporary and permanent hardness of water?
Answer: Temporary hardness of water is due to the presence of bicarbonates of calcium and magnesium in water i.e., Ca(HCO3)2 and Mg(HCO3) in water. Permanent hardness of water is due to the presence of soluble chlorides and sulphates of calcium and magnesium i.e., CaCl2, CaS04, MgCl2 and MgS04.
Question 23. Discuss the principle and method of softening of hard water by synthetic ion-exchange resins.
Answer: Cation exchange resins have large organic molecule with S03H group which are insoluble in water. Ion exchange resin (RS03H) is changed to RNa on treatment with NaCl. The resin exchange Na+ ions with Ca2+ and Mg2+ ions present in hard water and make it soft.
2RNa(s) + M2+(aq) ——> R2M(s) + 2Na+(aq)
where, M = Mg, Ca.
The resins can be regenerated by adding aqueous NaCl solution.


Question 28. Describe the usefulness of water in biosphere and biological systems.
Answer: (i) Major part of all living system is made of water.
(ii) It constitutes about 65 – 70% of body weights of animals and plants.
(iii) Some properties of water like high specific heat, thermal conductivity, surface tension, high polarity allow water to play a major role in biosphere.
(iv) Because of high heat of vaporisation it is responsible ro regulate temperature of living beings.
(v) It is an excellent fluid for the transportation of minerals and nutrients in plants.
(vi) It is also required for photosynthesis in plants.
Question 29. What properties of water make it useful as a solvent? What types of compound can it (i) dissolve (ii) hydrolyse?
Answer: Water is highly polar in nature thats why it has high dielectric constant and high dipole moment. Because of these properties, water is a universal solvent.
It can hydrolyse many oxides metallic or non-metallic, hydrides, carbides, nitrides etc.
Question 30. Knowing the properties of H2O and D2O, do you think D2O can be used for drinking purpose.
Answer: No, D2O is injurious to human beings, plants and animals.

Question 33. What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15,19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
Answer: Atomic No. 15 is of phosphorus. The hydride is PH3 and its nature is covalent. Atomic No. (Z = 19) is of potassium. The hydride is KH and it is ionic in nature. Atomic No. (Z = 23) is of vanadium. The hydride is VH. It is interstitial or metallic. Atomic No. 44 is of ruthenium, its hydride is interstitial or metallic.
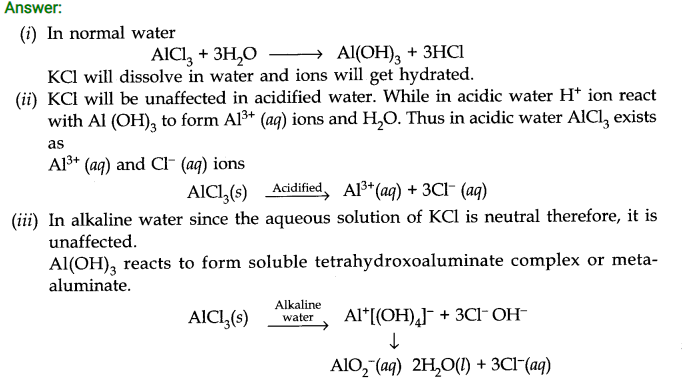
Question 34. Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified water (iii) alkaline water? Write equation wherever necessary.
Question 35. How does H2O2 behave as a bleaching agent?
Answer: Bleaching action of H2O2 is due to the oxidation of colouring matter by nascent oxygen.
H2O2(Z) ———> H2O(Z) + O(g)
Question 36. What do you understand by the terms:
(i) Hydrogen economy (ii) hydrogenation (iii) syngas (iv) water-gas shift reaction
(v) fuel-cell?
Answer: (i) Hydrogen economy: The basic principle of hydrogen economy is the storage and transportation of energy in the form of liquid or gaseous dihydrogen.
(ii) Hydrogenation: Hydrogenation means addition of hydrogen across double and triple bonds in presence of catalyst to form saturated compounds.