NCERT Solutions for Class 11 Chemistry Chapter 10 Very Short Answer Type Questions
Question 1. Name the alkali metal which shows diagonal relationship with magnesium?
Answer: Li.
Question 2. Why alkali and alkaline earth metals cannot be obtained by chemical reduction method?
Answer: Because alkali and alkaline earth metals are themselves stronger reducing agents than the majority of other reducing agents.
Question 3. Name the compounds used for the manufacture of washing soda by Solvay process.
Answer: NaCl, CaCO3 and NH3.
Question 4. Which electrolyte is used to obtain sodium in Castner’s process?
Answer: Fused NaOH.
Question 5. What happens when crystals of washing soda are exposed to air?
Answer: Monohydrate (Na2CO3– H2O) is formed as a result of efflorescence.
Question 6. Name the alkaline earth metals whose salt do not impart colour to a non-luminous flame.
Answer: Beryllium does not impart colour to a non-luminous flame.
Question 7. What is dead burnt plaster?
Answer: It is anhydrous calcium sulphate (CaSO4).
Question 8. What is Quick lime? What happens when it is added to water?
Answer: CaO is quick lime. When it is added to water, Ca(OH)2 is formed.
Question 9. Arrange the following in the increasing order of solubility in water.
MgCl2, CaCl2, SrCl2, BaCl2
Answer: BaCl2 < SrCl2 < CaCl2 <MgCl2
Question 10. Give the chemical formula of Epsom salt.
Answer: MgSO4,7H2O
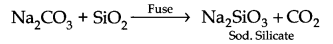
Question 11. How would you prepare sodium silicate from silica?
Answer:
Question 12. What happens when sodium metal is heated in free supply of air?
Answer: Sodium peroxide is formed.
2Na + O2 ——-> Na2O2
Question 13. What is the general name for elements of group 1 ?
Answer: Alkali metals.
Question 14. Why are alkali metals soft?
Answer: Since the atoms of alkali metals have bigger kernels and smaller number of valence electrons, the metallic bonds in them are very weak and hence are soft.
Question 15. What do you mean by diagonal relationship in periodic table?
Answer: The resemblance of the first element of second period with diagonally situated element of neighbouring element is called diagonal relationship.
Question 16. Why is BeCl2 soluble in organic solvent?
Answer: Since BeCl2 is a covalent compound it is soluble in organic solvent.
Question 17. Why do alkali metals give characteristic flame colouration?
Answer: Alkali metals due to low ionization energy absorb energy from visible region to radiate complementary colour.
Question 18. Why is the solution of alkali metals in liquid ammonia conducting in nature?
Answer: Due to ammoniated electrons and cations.
Question 19. Which is more basic NaOH or Mg(OH)2?
Answer: NaOH is more basic.
Question 20. Which alkaline earth metals do not impart colour to the flame?
Answer: Be and Mg.
Question 21. What is soda ash?
Answer: Soda ash is anhydrous sodium carbonate (Na2CO3).
NCERT Solutions for Class 11 Chemistry Chapter 10 Short Answer Type Questions
Question 1. Why are alkali metals always univalent? Which alkali metal ion forms largest hydrated ion in aqueous solution?
Answer: They are always univalent because after losing one electron, they aquire nearest inert gas configuration.Li+ forms largest hydrated cations because it has the highest hydration energy.
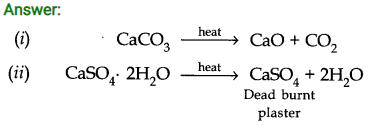
Question 2. What is the effect of heat on the following compounds (Give equations for the reactions)? (i) CaC03 (ii) CaSO4 2H2O
Question 3. Explain the following:
(a) Lithium iodide is more covalent than lithium fluoride.
(b) Lattice enthalpy of LiF is maximum among all the alkali metal halides.
Answer: (a) According to Fazan’s rule, Li+ ion can polarise l– ion more than the F– ion due to bigger size of the anion. Thus Lil– has more covalent character than LiF.
(b) Smaller the size (internuclear distance), more is the value of Lattice enthalpy since internuclear distance is expected to be least in the LiF.
Question 4. Write the chemical formula of the following compounds.
(i) Chile salt petre (ii) Marble (iii) Brine
Answer:(i) NaNO3 (ii) CaCO3 (iii) NaCl.
Question 5. Explain the following:
(a) Why Cs is considered as the most electropositive element?
(b) Lithium cannot be used in making photoelectric cells.
(c) Lithium does not form alums.
Answer: (a) Due to its lowest ionization energy, Cs is considered as the most electropositive element.
(b) Lithium cannot be used in making photoelectric cells because out of all the alkali metals it has highest ionization energy and thus cannot emit electrons when exposed to light.
(c) Due to small size, lithium does not form alums.
Question 6. (a) What makes lithium to show properties uncommon to the rest of the alkali metals?
(b) When is a cation highly polarising? Which alkali metal cation has the highest polarising power?
Answer: (a) The unusual properties of lithium as compared to other alkali metals is due to its exceptionally small size of atom and its ion and its high polarising power.
(b) A cation is highly polarising if its charge/ size ratio is very high.
Li+ ion has the highest polarising power.
Question 7. Why are ionic hydrides of only alkali metals and alkaline earth metals are known? Give two examples.
Answer: Alkali metals and alkaline earth metals are most electropositive due to low ionization ethalpy therefore they form ionic hydrides, e.g. NaH, KH and CaH2
Question 8. Why does the solution of alkali metals becomes blue in liquid ammonia? Give the chemical equation also.
Answer: The blue colour of the solution is due to ammoniated electron which absorbs energy in the visible region of light and imparts blue colour.
Na (am) + e- (am) + NH3(l) ——–> NaNH2(am) + —1/2 H2(g)
Question 9. Give the important uses of the following compounds.
(i) NaHCO3 (ii) NaOH
Answer:
(i) Uses of NaHCO3
- It is used in fire extinguisher.
- It is mild antiseptic for skin infections.
- It is used as antacid.
(ii)Uses of NaOH
- It is used in soap industry.
- It is used in textile industry.
- It is used as reagent in laboratory.
- It is used in absorbing poisonous gases.
Question 10. What is the mixture of CaC2 and N2 called? How is it prepared?
Answer: It is called Nitrolime.
It is prepared by heating CaC2 with N2 at high temperature.
CaC2 + N2 ——–> CaCN2 + C
NCERT Solutions for Class 11 Chemistry Chapter 10 Long Answer Type Questions
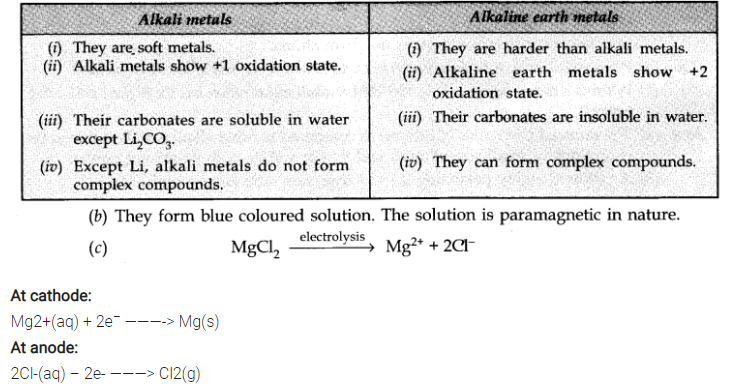
Question 1. (a) Compare four properties of alkali metals and alkaline earth metals.
(b) What happens when alkali metals are dissolved in ammonia?
(c) MgCl2 is electrolysed.
Answer: (a)
Question 2. State as to why
(a) Alkali metals show only +1 oxidation state. (b) Na and K impart colour to the flame but Mg does not.(c) Lithium on being heated in air mainly forms the monoxide and not the peroxide.(d) Li is the best reducing agent in aqueous solution.
Answer: (a) Alkali metals have low ionization enthalpies.
They have a strong tendency to lose 1 electron to form unipositive ions. Thus they show an oxidation state of +1 and are strongly electropositive.
(b) Valence electrons of alkali metals like Na and K easily absorb energy from the
flame and are excited to higher energy levels. When these electrons return to the ground state, the energy is emitted in the form of light.
Magnesium atom has small size so electrons are strongly bound to the nucleus. [ Thus they need large amount of energy for excitation of electrons to higher
energy levels which is not possible in bunsen flame.
(c)Due to the small size of Li+ it has a strong positive field which attracts the negative charge so strongly that it does not permit the oxide ion, 02- to combine with another oxygen atom to form peroxide ion.
(d)Since, among alkali metals, lithium has the most negative electrode potential (E° = -3.04 V) so, it is the strongest reducing agent in the aqueous solution.