
Physical Properties of group 14
- Atomic size: If we compare its size with group 13, then size of group 13 elements is bigger than group 14. As group 14 elements are smaller due to increased nuclear charge.
Along group: As we move down size increases, as each time a new shell is being added.
- Ionization energy: It is amount of energy required to remove electron from last shell of an atom ,when it is in gaseous state .We know ionization energy is inversely proportional to size therefore , ionization of group 14 is higher than group 13, due to its small size .
Along group: Ionization energy decreases because size increases. If we look at the trend of ionization energy we see certain abnormality:
C > Si > Ge >Sn <Pb
Carbon silicon germanium Tin lead
As we move from tin to lead ,the ionization energy increase due to poor shielding effect of 4 f orbital in Lead .
- Melting point and Boiling point
The boiling point of group 14 is higher than group 13 .As they form covalent bonds because of their small size.
As we move down the group melting and boiling point decreases due to increase in size, bonds formed are not so strong.
C > Si > Ge >Sn >Pb
Carbon silicon germanium Tin lead
4 .Metallic character
- The tendency to lose electrons depends upon ionization energy or, we can say that less is the ionization energy, more is the metallic character.
- If we compare for group 13 and group 14, we see that group 13 is more metallic due to big size and low ionization energy.
- Down the group metallic character increases as size increase and ionization energy decreases. Therefore, the order is :
- C Si Ge Sn Pb
- Carbon silicon germanium Tin lead
(non metals ) (metalloid ) (metals )
- Oxidation states
This group can show oxidation states +4 and +2.
- Carbon: Due to high ionization energy, sharing is preferred in case of it. So, oxidation state shown is 4.
- Silicon,Ge, Tin : They commonly show +4.
- Lead: Show +2 due to inert pair effect.
- All these elements have special property that is,if they are present in +2 oxidation state they act as reducing agents .
Example: Tin (Sn+2)

Chemical properties of group 14
They are non reactive but reactivity goes on increasing down the group due to decrease in ionization energy.
- Reactivity towards oxygen : They form two types of oxides
- Monoxides (MO)
- Dioxides (MO2)
That is :
- Monoxides : CO,SiO,GeO,SnO,PbO
- Dioxides: CO2,SiO2,GeO2,SnO2,PbO
Out of them:
- Co: Neutral
- SiO: is not so stable
- GeO: Weakly acidic
- SnO and PbO : Amphoteric
- CO2,SiO2 : Acidic
- GeO2: Amphoteric
- SnO2 and PbO2: weakly basic
Out of them, CO Is strongest reducing agent because it has ability to accept oxygen and form stable oxide that is carbon dioxide .The solid form of carbon dioxide is called dry ice and the commercial name of dry ice is drikold. Out of them PbO2 is strongest oxidizing agent .
- Reaction with water: They form hydroxides.
- In this group, Carbon does not react with water.
- Tin reacts with steam forming SnO2+H
- Ge,Sn Pb –do not react with water due to formation of protective layer of oxide on it .
- Reaction with halogens: Halides are formed (EX4).
The halides formed are :
CCl4SiCl4 GeCl4 SnCl4 PbCl4
SnCl2 PbCl2
All are tetrahedral in nature.
Structure of CCl4:

- Reaction with hydrogen : hydrides are formed (EH4)
They form respective hydrides:
- CH4 SiH4 GeH4 Sn and Pb do not form as they are less reactive towards hydrogen.
- Carbon has maximum tendency to form hydrides in its own family .these hydrides have covalent bonding in them and a tetrahedral geometry.
Allotropes of carbon
Allotropes: Are the different forms of elements having same physical properties but different chemical properties.
Allotropes of carbon
- Crystalline form :Diamond ,Graphite and Fullerene
- Amorphous forms of carbon : Coke ,Charcoal ,lamp black
- Diamond
In this carbon is sp3 hybridized .Each carbon attached to four carbon atom giving rise to compact three dimensional structures given below:
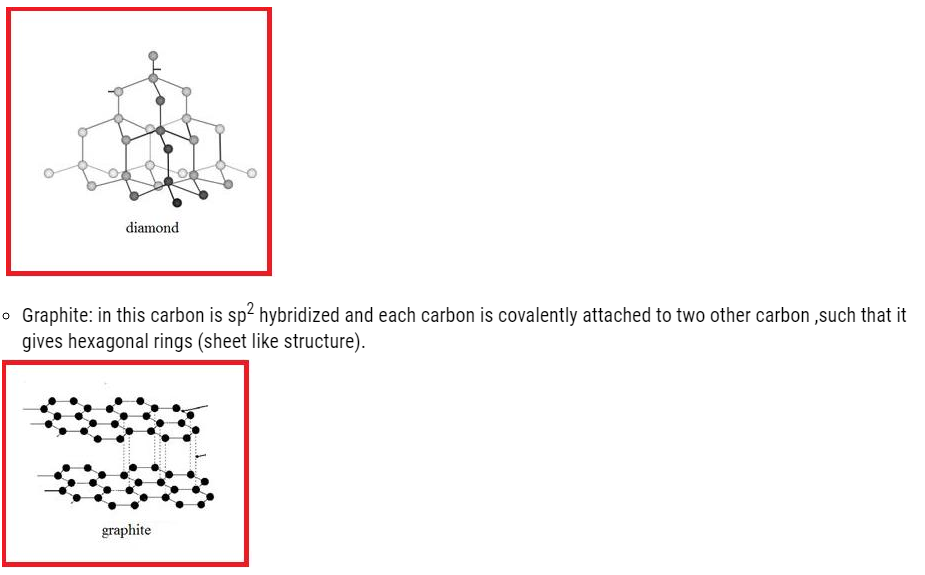
- The layers are held by weak Vander wall forces, such that they can slide over one another.
Properties and Uses of diamond and graphite
- Diamond
- It is hardest substance. Therefore, used as cutting tool.
- It is bad conductor of electricity because it has no free electrons.
- Graphite: It has soft structure because of Vander wall forces in it.
- Therefore, used as Lubricant.
- It is used to make pencil leads, as it marks the paper black.
- Moreover, it is good conductor of electricity as it has free electrons.
Uses of Diamond
- It is used as cutting tool.
- It is used in making jewellery.
- It is used in manufacturing of tungsten filament.
Uses of Graphite
- It is making electrodes.
- It is used as lubricant
- It is mixed with clay or wax to make lead pencils
- It is used to making moderator of nuclear reactor.
Both Diamond and Graphite are crystalline forms of Carbon.
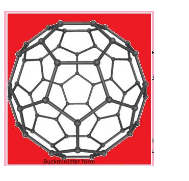
- Fullerenes: It is having many Carbon atoms ranging from C32 to CIt was studied in 1985 and after 10 years it came in notice or structure. The carbon atoms are in a shape of football .
Buckminster form :
- Amorphous forms of Carbon : In this the molecules are arranged in haphazard manner .
- Coke: Used as fuel.
- Charcoal : Is porous
Types of Charcoal:
- Wood charcoal
- Animal charcoal
- Sugar charcoal
(They are obtained by destructive distillation of wood, sugar etc).
- Charcoal is good absorbent, if dipped in colored solution it will adsorb all colours, leaving behind colorless solution.
Uses of Carbon
- It is used in the form of fuel.
- It is used in manufacturing of coal gas, water gas etc.
- It is also used as a good reducing agent in metallurgy.
- It is activated charcoal and is used as catalyst.
Uses of Silicon
- It is used to form n-type or p-type semiconductor.
- It is important component of glass and cement.
- Pure Si is used to make computer chips.
Uses of germanium
- It is used in transistors.
- It is making for lenses and prism.
- It is used as scientific apparatus.
Uses of lead
- It is used for making lead sheets and pipes.
- It is used for telephone wires.
- It is used in storage batteries and bullets.
Uses of Tin
- It is used for electroplating.
- It is used in making alloys: Pb, Cu and Sn.
- It is a type metal: Pb ,Sn ,Sb.