ELECTRON DISPLACEMENT IN COVALENT BONDS
Whenever any reaction occurs, old bonds break and new bonds are formed. This formation is due to shifting of electrons. There are four types of electron displacements:
- Inductive effect
It is a permanent effect that arises when some electron releasing or withdrawing group is attached to chain of alkane.
Example:- C-C-C-C-C1-X
- If X is electronegative, it will attract the shared pair electrons more towards itself. As a result, X will acquire partial negative charge and C1 will acquire partial positive charge.
- Now this positive charge of C1 will attract electrons of C1 – C2 As a result, some of the positive charge on C1 will be reduced and on C2 it will develop.
- This effect happens only till 4thcarbon as after 4th carbon the magnitude of charge is almost nil.
- So, we can define it as “the displacement of electrons along the saturated carbon chain due to presence of polar covalent bond at the end of the chain. “
It is of two types
- -I effect
- +I effect
-I effect –It is shown when electron withdrawing group is attached to the carbon chain.
- Please note: More is the electron withdrawing, more is the -I effect.
- Order of groups in increasing strength towards -I effect:
NO2 > CN> COOH > COO> F> Cl> Br> I
+I effect – It is shown when electron releasing group is attached to carbon chain .
Example – Alkyl groups.
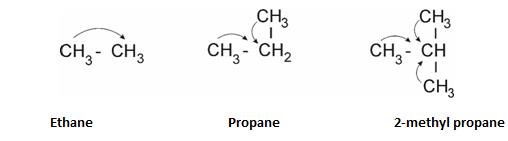
- “More the number of alkyl groups , more is the +I effect.”
- Order of strength: 30> 20> 10
2. Electrometric effect
- It is a temporary effect that is shown in the presence of attacking agent.
- It is shown by multiple bonded systems like C=C , C=O
- It is of two 2 types :
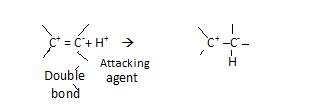
+E effect –
It is that effect when electron of π bond is transferred to atoms to which attacking agent attaches itself.
-E effect –
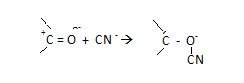
“It is that effect when π electrons are transferred to atom other
than the atom, to which attacking agent finally attaches itself.”
- Resonance
It occurs due to delocalisation of π electron cloud so as to get best structure as compared to other structure.
Example: Structure of CO2

But, it was seen that Bond Length of that CO bond is not same as that of C=O. It lies in between C-C and C O. So, as to explain the Resonating structures were proposed.
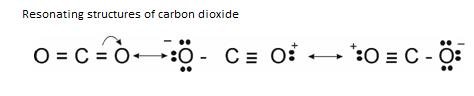

Resonance is best seen in Benzene: It is also called as Mesomeric effect .It arises due to polarity developed in Benzene ring due to delocalisation of π electron.
+ R effect –It is shown when electron donating group is attached to benzene ring. Like
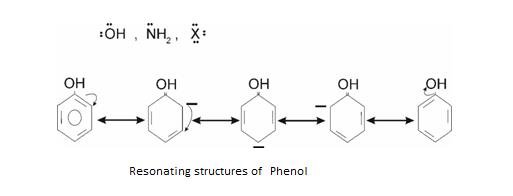
If any positively charged group approaches it attacks at Ortho and Para positions.
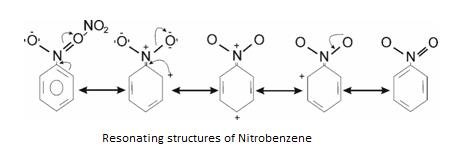
-R effect – Shown by electron withdrawing group when attached to benzene ring.Example:
HYPER CONJUGATION
It is like resonance but the difference is that there is no bond present from where the electrons are shifted.
The Condition required: It arises when = and – bond is at adjacent position.
Example: –:
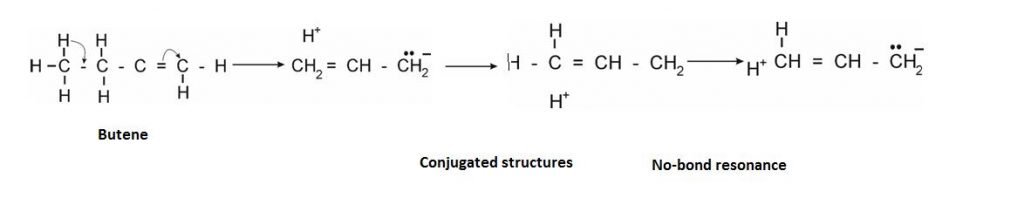
- “More the terminal Hydrogen , more is the Hyper-conjugated structure.”
- We can define it as interaction between π electrons and adjacent bond of substituent group attached to it.
- It is also called as Baker Nathan and non-bond resonance.
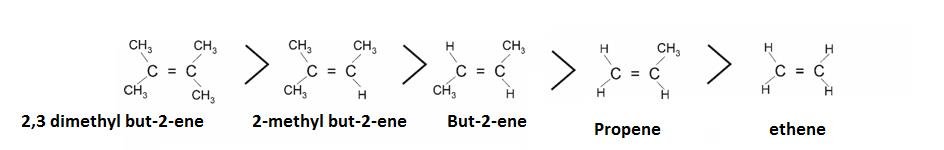
- It can explain stability of Alkylated alkenes.
Fundamental concepts of organic reactions
Whenever a chemical reaction takes place the old bonds break and new bonds of products is formed.
- Breaking of bond — fission.
- Formation of bonds — products formed.
- Bond fission is of 2 types:
- Homolytic
- Heterolytic
Homolytic fission: The bond breaks in such a way that each electron of shared pair is taken equally by each atom.
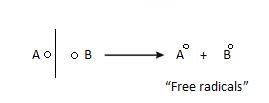
Heterolytic fission: The bond break in such a way, that one species takes
both the electrons of shared pair and other remain deficient of shared pair.
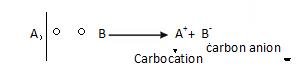
REACTION INTERMEDIATES
Reaction intermediates are free carbocation and carbon anion.
- Free Radical: It formed as a result of homolytic fission. Due to this extra electron, they are quite reactive.
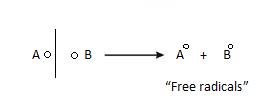
They are of 4 types:

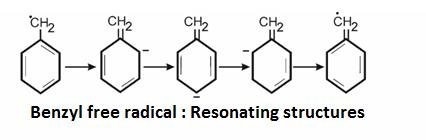
Stability of these radicals:
30> 20> 10> CH3
We can explain their stability by :
30> 20> 10> CH3
It is explained on the basis of hyper configuration: More the alkyl groups , more is the number of hydrogen and more are the conjugated structures .Hence, more is the stability.
- Carbocation: It is formed as a result of hydrolytic fission. It is the Carbon with six electron and a positive charge.
