Aromatic Compounds ( For Detail Refer Book )
- It means aroma (pleasant smell).
- They have benzene ring C6 H6 (alternate 3 double bond with no benzene ring).
- All with benzene ring is called are called Benzenoid compound and with no benzene ring is called non-benzenoid compound).
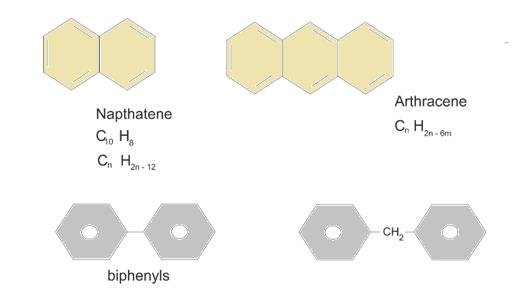
When 2 benzene rings on fused:
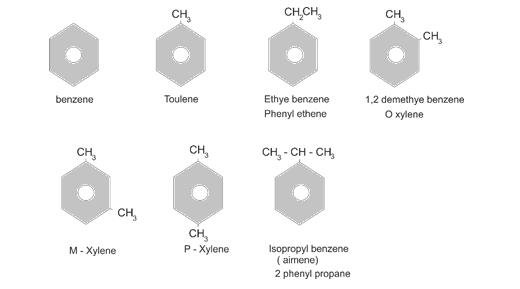
Nomenclature
Isomerism: Ortho, Meta and Para isomers are formed. ( See In Book )
Stability and structure of benzene ( See In Book )
- In benzene the double bonds keep on moving so, the structure showing double bond can be written in either ways. It has no effect on the chemical reactions of benzene.
Or we can say that pi electrons keeps moving i.e. shifting of π electron takes place. Therefore basic structure of Benzene can be written as shown below .The Hybridisation is sp 2.
__ ( Benzene )
- Structure of benzene is planar with sp 2 hybridisation .
- To see stability we can calculate enthalpy of hydrogenation.
The difference is resonance energy is:
i.e. 358.5 – 208.1
= 150.4 KJ/mol
So, we conclude that benzene is more stable than normal alkene.
Huckle rule of Aromatic compounds
It gives criteria of aromaticity that is:
- Delocalization of electron.
- All aromatic compound should have (4n + 2 ) π electrons , Where n = 2 , 6, 10 , or 14.
Example:
Preparation of Benzene
- Polymerisation of alkynes: In this the 3 molecules of alkynes undergo polymerisation as shown in presence of iron at temperature approximately 873 K.
(Benzene)
- Decarboxylation of aromatic acids: The reaction involved is given below.
- Reduction of phenol: In this reduction of phenol is carried in presence of zinc on heating as given.
- Field craft alkylation – This reaction involves introduction of alkyl group in benzene.
Physical properties of benzene
- Existence: Benzene and its homologous (Benzene with side chain) up to 8 C atoms are colourless liquid while higher ones are solid with characterized smell.
- Solubility: They are insoluble in water but soluble in organic solvent.
- Flammability: They are inflammable and burn with sooty smell.
- Melting point and boiling point: It increases with increase in number of carbon atoms in chain.
- Nature: They are toxic and carcinogenic in nature.
Chemical properties of benzene
- Substitution Reaction – In this hydrogen is going to get substituted by some other group.
- Halogenation
The Mechanism involved is electrophilic substitution mechanism.
Ist step: Formation of electrophile
2nd step: Attack of electrophile on benzene ring
- Order of halogenations : For fluorine it is quite fast and also leads to explosion
F2 > Cl2> Br2> I2
3rd step: Deprotonation
- Sulphonation (substitution by SO3 H group)
Nitration (substitution by nitro group)
- Alkylation (substitution by alkyl group)
- If we want to get 6 halo product then the following reaction occur.
- Addition Reaction: It involves the addition of group or group of atoms to benzene.
- Hydrogenation
- Halogenation
- Addition of ozone molecule
- Oxidation Reaction
It can be complete oxidation or oxidation of alkyl chain with mild oxidising agent or strong oxidising agents.
- Combustion (Complete oxidation)
- Controlled oxidation in presence of vanadium penta-oxide.
- Oxidation of alkyl chain
With mild Oxidising agent
With strong oxidising agent: Etard reaction.