Green House Effect
Global warming
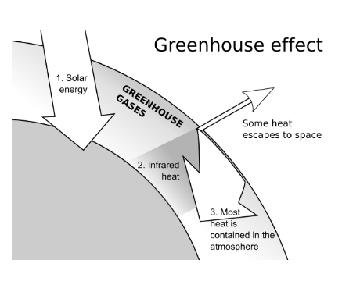
Global warming “is the increase in the concentration of certain gases like carbon dioxide, methane, etc. in air due to which infra-red rays get trapped in it and it leads to increase in overall temperature of earth.”
- The rays that come from the Sun consist of visible light, infra-red rays and ultra violet rays. Out of these, only infra-red rays and visible light reaches earth’s surface. On reaching earth, the infra-red rays are reflected back and a portion of it is absorbed by earth’s surface. The reflected ones when pass through earth’s atmosphere, get trapped inside the gas molecules. As we know, itleads to heating effect. Therefore, the overall temperature of earth’s surface rises. One of the reasons for global warming is greenhouse effect.
- In winters, it is advised to keep plants in green houses only. The roof allows infra-red rays to enter but after reflection, they are not allowed to go back. As a result, they get trapped inside the green house and growth of plants is stimulated because optimum temperature is achieved inside the green house.
Oxides of Sulphur:They are produced by burning of Sulphurcontaining fossil fuels. This Sulphur dioxide can cause respiratory problems like asthma, emphysema, irritation to eyes, redness in eyes etc.
Oxides of nitrogen: The nitrogen oxides are formed due to lightning strikes at higher altitudes and also due to burning of fossil fuels.It causes irritant red haze, damage leaves of plant, retard rate of photosynthesis also respiratory disorders.
Both Sulphur dioxide and nitrogen dioxide are cause of acid rain
Acid rain ( Environmental Chemistry )
- It is the one of the major causes of causing cancer. It is formed when rain water mixes with pollutants present in air. These pollutants are mainly Nitrogen dioxide and Sulphur dioxide gas emitted in exhaust of vehicles.
When rain falls, these gases combine and the following reaction takes place:
SO2 + H2O H2SO4
NO2+ H2O HNO3
As a result, rain water no longer remains only pure water it becomes acidic
Harmful effects:
- It causes cancer.
- It combines with marble and deteriorates the marble.
- It affects ph. of soil and makes it unfit for growing plants.
- It affects aquatic life too
Hydrocarbons:They are produced due to combustion of fuels are actually carcinogenic that causes cancer. They also harm plants as they cause ageing in plants, shedding of leaves etc.
Chlorofluorocarbons:They are released into the atmosphere from industries. They are actually manmade industrial chemicals used in air conditioners, refrigeratorsetc. and their vapours do not remain in lower layers of atmosphere they move up and cause thinning of ozone.
