Alkynes
In this triple bond is present between carbon atoms (C C bond).The hybridisation is sp and the general formula involved is CnH2n -2.
- The lowest member of alkynes is: simplest compound that is CH CH (ethyne).
- The common name of it is acetylene.
- It has high Bond dissociation energy, as there is triple bond present therefore the energy is high of the order 823 KJ/mol.
- Due to this reason the acetylene is mixed with O2 and the flame is used for welding purpose.
Structure of ethyne
The geometry is linear structure with bond angle 1800 and sp hybridization.

Nomenclature: Suffix used is “yne”
CH3 – CH2 -CH2-C CH CH3-C C-CH3
Pentyne but – 2 – yne
CH2= CH -CH2– C CH
Pent – 1 – en – 4 yne
Isomerism: There is restricted rotation around C C bond therefore no geometrical isomerism is seen. .
- Chain isomerism (converting straight chain to branched).
CH3 -CH2-CH2CH2 – C CH
Hexyne
CH3– CH2– CH(CH3) – C CH
3 methyl pent – 1 – yne
- Position isomerism (change in the position of triple bond).
CH3– CH2-CH2 -CH2 -C CH CH3 – CH2-C C-CH2– CH3
Hexyne Hex – 3 – yne
- Functional isomerism: It involves conversion of one triple bond to two double bonds.
CH3 CH2– C CH CH2 = CH- CH = CH2
(C4 H6) (C4 H6) same molecular formula
Ring Chain Isomerism: In this Open chain is converted to ring structure.
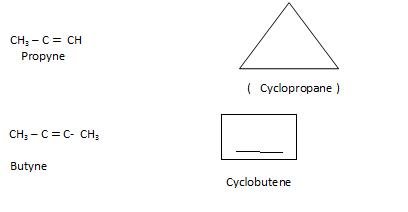
Preparation of Alkynes
- Laboratory preparation –It is prepared in by action of H2O on Calcium carbide i.e. CaC

- First we need to prepare Calcium carbide CaC
CaO + 3C à CaC2 + Co
Lime (273K)
The reaction involved is:

This reaction can lead to explosion so, in order To avoid explosion we need to remove air and for that we take oil gas.
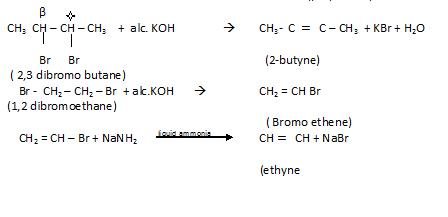
- By dehydrohdogenation of vicinal dihalide
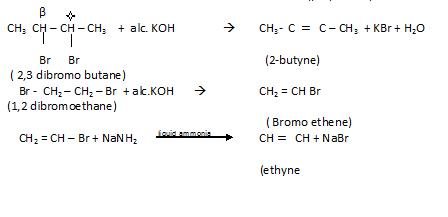
By action of Zinc on Tetra halogen derivatives

We can prepare higher alkyne from acetylene: This is also called up-gradation reaction.
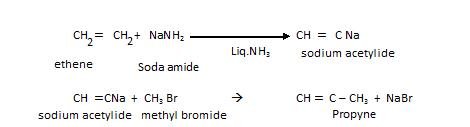
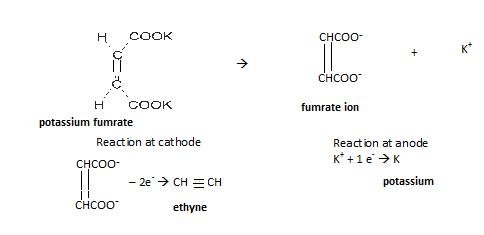
- Kolbe’s electrolytic reduction
In this electric current is passed through metal carboxylic salt as shown:
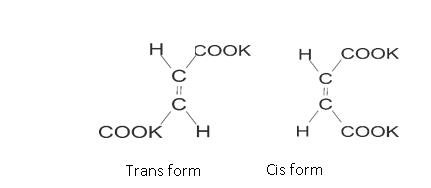
It can be in cis form or trans form:
Reaction involved:
Dehalogenation of haloforms: In this 2molecules of chloroform reacts with silver to form alkyne.

7. Synthesis from Carbon & Hydrogen: In this electric discharge is passed through carbon and hydrogen mixture to form alkynes.

Physical properties of alkynes
1. Existence: Acetylene, propyne, butyne are gases and other with carbon 4 to 12 are liquids, more than 12 are solids.
C4 – C12 liquids
> C12 solids.
- They all have no smell, except garlic like smell of acetylene.
- Solubility: They are weekly polar in nature and not soluble in H2O but soluble in organic solvents.
- Melting point and boiling point: Because of triple bond they have higher melting point and boiling point.
- With increase in number of carbon atoms the boiling point increases.
- With increase in branching the boiling point decreases.
Chemical properties of alkynes
- Acidic Character – This is shown by replacement of one of the hydrogen atom by metal atom.

Alkynes are acidic because of sp hybridisation of Carbon atom. The “s” character in case of alkynes is 50%. Due to more “s” character, electrons in sp hybrid orbitals are held more tightly by nucleus and are quite electronegative. As a result, the H – C bond get more displaced towards carbon atom and proton is easily released. Due to this, the bond easily breaks and the Hydrogen is easily released.
Application: By this we can upgrade number of carbon atoms in alkynes.
- Addition reaction: The basic general reaction involved in addition is similar to that of alkenes as shown.
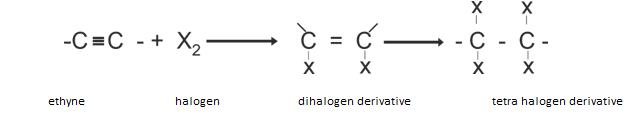
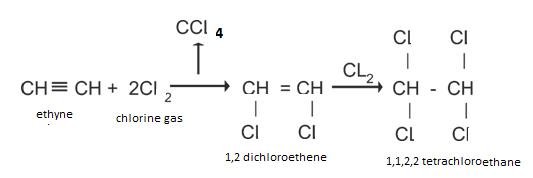
Addition of halogens
- Addition of halogen acids (hydro halogenations Reaction ) : The order of reactivity of this reaction is:
HF < HCl < HBr < HI
The reaction involved is:
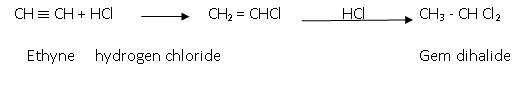

Anti-markoniov’s rule is followed in case of addition of HBr:
- Addition of H2O ( hydration Reaction)

Addition of HCN

Addition of H2

Please note:
When we use lindlar catalyst that is Pd/BaSO4 we get Cis form and whenever we use catalyst that is Na in liquid NH3, then it is called birch reduction and we get trans form as shown below :

- Oxidation of alkynes
The oxidation is carried out with:
- Cold and dilute alkaline potassium permanganate
- Hot and concentrated potassium permanganate
- With ozone
With Hot and Concentrated KMnO4

Please note that if we carryout oxidation of butyne with acidic potassium permanganate under low and high temperature we get different products as shown below:

With Ozone

Polymerisation
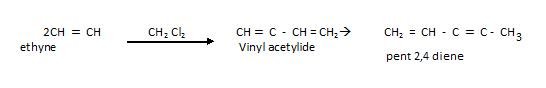
Cyclic Polymerisation
