Avogadro Law
- It states that equal volumes of all gases under the same conditions of temperature and pressure
contain equal number of molecules. Mathematically,
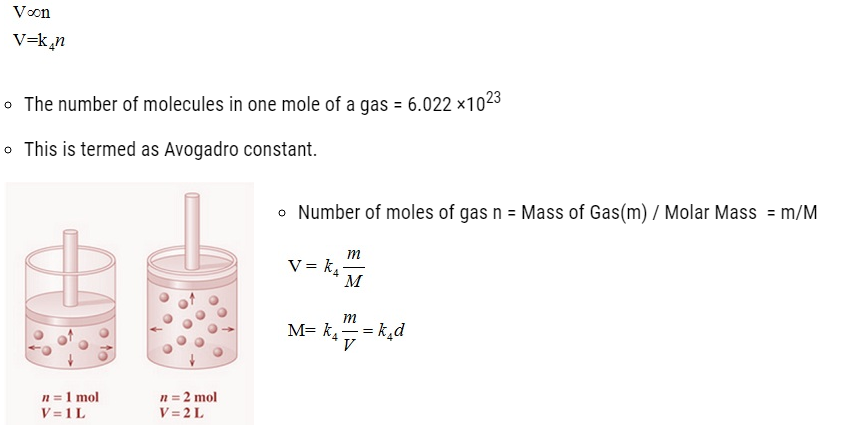
Ideal Gas Equation
- The gases following Boyle’s law, Charles’law and Avogadro law firmly are termed as anideal gas.
- From Boyle’s law we have V ∝ 1/p (Constant T, n)
- From Charles’ law we have V ∝ T (Constant p, n)
- From Avogadro’s law we have V ∝ n (Constant T, n)
- Combining them we get, V ∝ nT/p
- or V = nRT/p
pV = nRT ………………………… (IDEAL GAS EQUATION)
R =pV/nT
R= Gas Constant. It is same for all gases.
Problem:
Using the equation of state pV = nRT; show that at a given temperature density of a gas is proportional to gas pressure.
Solution:
Using Ideal gas equation, pV = nRT ……….. (1)
p → Pressure of gas
V → Volume of gas
n→ Number of moles of gas
R → Gas constant
T → Temperature of gas
From equation (1),
p = n RT/V
n = Mass of Gas (m) /Molar Mass of gas (M)
Putting value of n in the equation, we have
p = m RT/ MV ————(2)
Density (ρ) = m /V —————-(3)
Putting (3) in (2) we get
p = ρ RT / M
Or ρ = PM / RT
Problem:
Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm3 at 27°C. R = 0.083 bar dm3 K–1 mol–1.
Solution:
m (Oxygen) = 8 g,
M (Oxygen) = 32 g/mol
m ( Hydrogen) = 4 g,
M (Hydrogen) = 2 g/mol
n (Amount of oxygen) = 8/ 32 = 0.25 mol
n (Amount of hydrogen) = 4/2 = 2 mol
According to ideal gas equation,
PV = n RT,
P X 1 = (0.25 + 2) X 0.083 X 300 = 56.02 bar
Total pressure of the mixture is 56.02 bars.
Problem:
Calculate the volume occupied by 8.8 g of CO2 at 31.1°C and 1 bar pressure. R = 0.083 bar L K–1 mol-1.
Solution:
According to ideal gas equation, PV = nRT
But n = Mass of Gas (m)/ Molar mass of Gas (M)
PV = (m/M) RT
For CO2 , M = 44 g/mol
Putting the values
1 x V = (8.8 / 44) x 0.083 x 304.1
= 5.05 L
Volume occupied is 5.05 L.
Density and Molar Mass of a Gaseous Substance
- As per ideal gas equation,
pV = nRT
Or n/V = p/RT
- As we know n = Mass of Gas (m) / Molar Mass of Gas (M) = m/M
So, m/MV = p/RT
d/M= p/RT
- Or Molar mass = M = dRT/p
Problem:
At 0°C, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Solution:
Using the formula ρ = Mp/RT
or p = ρ RT/ M
According to the question,
For certain oxide gas
2 = ρ RT/ M ……………(1)
For nitrogen
5 = ρ RT/28 ………………….(2)
From equation (1) & (2), we get
5/2 = M/28
Or
M = 5 X 28/ 2 = 70g/mol
Problem:
Density of a gas is found to be 5.46 g/dm3 at 27 °C at 2 bar pressure. What will be its density at STP?
Solution:
According to the ideal gas
Density ρ = p x M/ RT
According to the question,
5.46 = 2 x M/ 300 x R ……… (1)
ρSTP = 1 x M/ 273 x R ……….. (2)
From equation (1) & (2),
ρSTP = (1 x M/ 273 x R) x (300 x R/ 2 x M) = 300 / 273 X 2 = 3.00 g/dm3
Density of the gas at STP will be 3 g dm–3.
Problem:
Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Solution:
m ( Nitrogen) = 1.4 g
M (Nitrogen) = 28 g mol–1
n (Amount of nitrogen) = 1.4 / 28 = 0.05 mol
Hence number of nitrogen molecule in 0.05 mol = 6.023 x 1023 x 0.05 = 3.0115 x 1022
Number of electrons in one molecule of nitrogen = 14
Total number of electrons in 1.4 g of nitrogen = 3.0115 x 1022 x 14 = 4.22 x 1023