Chemical properties of group 13
- Out of all elements of this group, Boron is non-reactive. This is because of its small size as it has high ionization energy .So, reactivity increases down the group.
B < Al < Ga < In < Tl
Boron aluminum Gallium indium thallium
- In this Boron is least reactive because of its small size and high ionization energy and Thallium is most reactive because of its low ionization energy.
- Aluminum: When react initially, it keeps reacting .But after some time, a layer of its oxide is formed over its surface. This layer prevent it from further reacting .So, it becomes passive after some time.
- Most of the compounds of this group are electron deficient that is their octet is not complete. So, they behave as Lewis acids.
Example: BX3 (only 6 electrons in valence shell of Boron).
Therefore, it forms coordinate bond.
BX3 + NH3–>[NH3–>BF3]coordinate bond
Boron halide ammonia
- That means, they have tendency to take electrons. Or, we can say they are Lewis acids. But down the group, acidic character decreases (that is tendency to attract incoming electron decreases).
BX3> AlX3 > GaX3 >InX3 >TlX3
boron halide aluminum halide gallium halide indium halide thallium halide
- Lewis acid strength decreases because size increases .As a result, attraction towards incoming electron decreases.
- Reactivity towards oxygen : Forms oxides
- Boron does not react with oxygen at ordinary temperature due to small size and high ionization energy.
- If we react Al with oxygen, it reacts at normal temperature. With time it forms a protective layer of oxide on its surface. This layer makes it non reactive.
Al +3O2 –>2Al2O3
aluminum oxygen aluminum oxide
- They react with nitrogen gas also, to form compound with formula EN.
Example: 6Al +3N2–>6AlN
aluminum nitrogen aluminum nitride
- If we see acidic strength of oxides, then it decreases down the group.
BeO Al2O3 GaO InO TlO
beryllium oxide aluminum oxide gallium oxide indium oxide thallium oxide
(acidic) (amphoteric) (all are basic)
- Reaction with water: Hydroxides are formed of type E(OH)
- Boron does not react with water.
- Aluminum reacts with cold water that is :
Al + H2O –> Al(OH)3 + H2
Aluminium water aluminium hydroxide hydrogen gas
- Gallium and indium neither react with cold water nor with hot water.
- Thallium reacts with water but form protective layer which make it passive.
- 3. Reactivity towards acids and bases:
- Boron doesn’t react with acids and bases at normal temperature, but reacts with strong acids.
- Aluminum reacts with acid and base because it is amphoteric in nature.
Al + HCl–>AlCl3 + H2
aluminum hydrogen chloride aluminum chloride hydrogen gas
Al + NaOH –>[NaAl(OH)4]
Aluminum sodium hydroxide sodium tetrahydroxoaluminate
- Al when react with nitric acid, initially it reacts but after same time it became passive.
- Reaction with Halogens: Form halides of type EX3
2B +3X2–>2BX3
boron halogen boron halide
B +F2 –>BF3
boron halogen boron halide
Al +Cl2àAlCl3
aluminum chlorine aluminum chloride
All halides are Lewis bases.
Important trends
- Formation of Hydrides : EH3
- Thermal stability of hydrides decrease down the group .Out of all, only BH3 is stable.
BnHn+4 , BnHn+6= Boranes
Simplest Borane :B2H6(diborane )
- This diborane has banana bond and it is weak Lewis acid.
- Formation of Halides: They form their respective halides. Out of all halides aluminum chloride AlCl3exist as dimer that is Al2Cl6 (all halides behave as Lewis acids)
- They will behave as Lewis acids only, if they attract incoming electrons.
- Trends of strength of Lewis acids :
- BF3>AlCl3>BBr3>BI3(expected order)
- BF3<AlCl3>BBr3>BI3(actual)
Explained on the basis of back bonding:
- In case of BF3, in Boron there is one vacant 2p orbital ( i.e 2s2,2p1)in ground state.
- In excited state, it will be 2s1, 2p2 and energy of 2p orbital of B and F are almost similar.
- As a result, one of the 2p filled orbital overlaps sidewise with the vacant 2p orbital of boron atom resulting in transfer of electrons from Fluorine to vacant 2p orbital.
- This is called back bonding.
- Now it doesn’t have fewer electrons therefore, Boron no longer act as a Lewis acid.
- As size increases, the tendency of back bonding decrease and acidic strength increase.
Uses of Boron
- Boron is used as semi conductor for making electrical appliances.
- It is used in steel industry for hardening.
- Its compounds like borax and boric acid are used in glass industry.
- Borax is used for soldering metals.
- Borax fibers are used in making bullets.
Uses of Aluminum
- It is soft and light metal, non toxic and is used for wrapping food items.
- It I used in making electric power cables.
- It is used as packaging of food items.
- It is used I making cans for cold drink etc.
Alloys of Aluminum
- Bronze : Aluminum and Copper are its constituents .It is used for making coins ,jewellery etc
- Magnalium: its constituents are Al and Mg.
It is used for making pressure cookers, balance beams etc.
- Duralumin: its constituents are: Al, Cu, Mg and Mn.
It is used for making bodies of air craft’s, helicopters, ships etc.
- ALNICO: Its constituents are AL, Ni and Cobalt.
It is used in making powerful magnets.
Borax
- Borax: Na2B4O7.10H2O

Preparation
- From Colemanite ore: It is prepared from Colemanite ore (calcium ore).
- In this ore is made to react with sodium carbonate.
- Then it is heated as shown in reaction :
Ca2B6O11 + Na2CO3—> CaCO3 +Na2B4O7 + NaBO2
Colemanite ore sodium carbonate calcium carbonate Borax sodium metaborate
- The solution has White precipitate.
- When these precipitates are filtered, the solution becomes concentrated.
- Finally, we get crystals of borax on cooling.
- From Boric acid : In this also boric acid is made to react with Sodium carbonate to form Borax ,carbon dioxide and water as shown below:
H3BO3 + Na2CO3—>Na2B4O7 + CO2 + H2O
Boric acid sodium carbonate borax carbon dioxide water
PROPERTIES OF BORAX
- It is white crystalline solid.
- On heating, it loses water of crystallization and form Na2B4O
- On further heating, it gives white transparent liquid which further on cooling gives white transparent bead.
- This bead is made to react with different types of salts.
- When reacted ,it gives different colour with different metal ions like :
With Ni2+–>brown
With Co2+–>blue
With Cr3+àgreen
With Mn2+–>pink
With Cu2+–>blue
Uses of Borax
- It is used in laboratory as Borax bead test.
- It is used in making enamels for pottery.
- It is used in candle making.
- It is added in soaps due to its antiseptic properties.
- It is used in optical glass.
- Ortho boric acid
Chemical Formula :- H3BO3 or B(OH)3
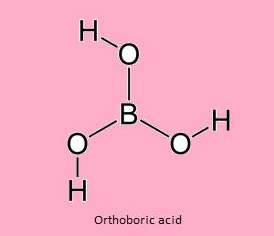
Preparation: It is prepared from Borax.
Na2B4O7.10H2O + HCl +H2O–>H3BO3 + NaCl
Borax hydrochloric acid boric acid sodium chloride
Properties
- It is white crystalline solid with soapy touch.
- It is sparingly soluble in cold water but soluble in hot water.
- At 373k, it forms metaboric acid.

- Boron hydrides
The molecular formula is BnH2n+4
The common hydride is :B2H6 that is diborane.
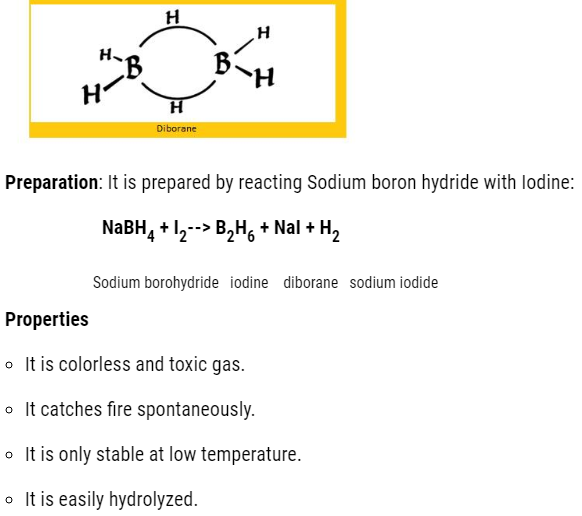
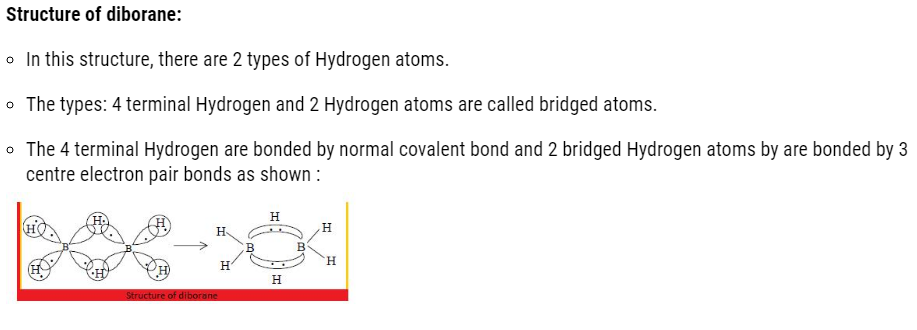
- Both the Boron will have in total 2 empty d orbital.
- Each 2p orbital overlaps with 1s orbital of H atoms.
- The 2 hybrid orbital left on each Boron atom contain an unpaired electron and other is empty.
- The orbital containing one electron of one boron atom and the other empty orbital of second boron atom form a bond with hydrogen atom simultaneously to give B-H-B bond.
- Each Boron form 2 covalent bond.
- Out of 3 unpaired electrons, the left electron of both the boron is 2 and of 2 Hydrogen atom is also 2.
- Therefore, it forms 3 bonds instead of 2 bonds.