Partial pressure in terms of mole fraction
- Let three gases be enclosed at
T = temperature three gases,
V = volume,
p1, p2 and p3 = partial pressure exerted on three gases respectively.
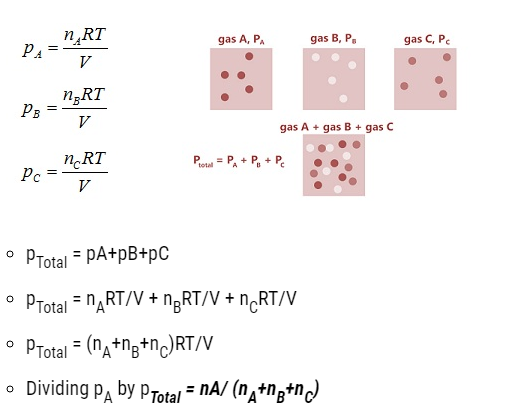
- PA = xA pTotal
Similarly, pB = xB pTotal
PC = xC pTotal
Problem:
A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
Solution:
Pressure of the gas mixture = 1 bar
Let the amount of mixture = 100g
Mass of hydrogen in mixture = 20 g
Mass of oxygen in mixture = 80 g
nH = 20/ 2 = 10 mol
nO = 80 / 32 = 2.5 mol
Using the formula,
pH = XH x Ptotal = (nH / nH + nO) x P total = (10 / 10 + 2.5 ) x 1
= 0.8 bar
Kinetic molecular theory of gases
- Molecules are point masses having no volume.
- Gas atoms apply no constrain on different atoms unless they suffer collision.
- Collisions of particles with each other or with the boundaries of container do not result in decreased energy system.
- The atoms of a gas are in consistent and irregular movement.
- The temperature of a gas relies upon its average kinetic energy 1/2 mv2 = 3/2 KT
- Ideal gas possesses kinetic energy.
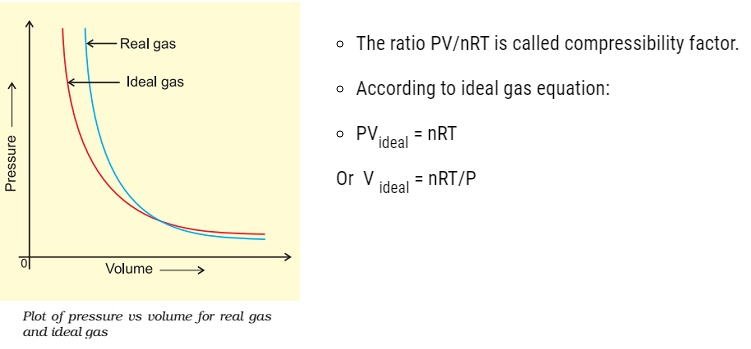
Behaviour of real gases: deviation from ideal gas behaviour
- The isotherm obtained in the graph by plotting the pressure (P) vs Volume (V) for real gas does not coincide with the slope of ideal gas.