DUAL NATURE OF LIGHT: DEVELOPMENT LEADING TO BOHR’S MODEL ( Structure of Atom )
There were so many factors, phenomenon that lead to the failure of Rutherford model:
- Dual nature of matter
- Atomic spectra
But before studying Bohr model, we need to learn electromagnetic radiations.
Newton said the corpuscles of light possess wave nature of light.
It was able to explain reflection, refraction etc. But failed to explain phenomenon of interface or diffraction. So, particle nature of light was considered.
Let us study about dual nature. It is electromagnetic theory, which was given by Huygens.This proved wave like character of
Light and tells us about wave motion. Wave motion is like if you throw stones, you will see a ripple that is wave.
Electromagnetic theory: According to this theory
- Energy is emitted or absorbed continuously in the form of radiant energy.
- These radiations consist of electric and magnetic field acting perpendicular to the direction of propagation of wave.
- These waves can travel through medium as well as through vacuum.
- The radiations travel with speed =3×108m/sec.

- Frequency: number of waves produced in one second.
- Units Used:- sec-1, Hertz
- Velocity of light: It is distance travelled with respect to time in any direction.
- Units Used:- m/sec
- Amplitude: maximum displacement of particle from its mean position.
- Units Used:- m or cm
- Wave number: number of waves in 1cm length.
Black body radiation and Photoelectric effect ( Structure of Atom )
Particle nature of Electromagnetic radiations :There were two important phenomenon that couldn’t be explained by considering Light with wave character:
The phenomenon is:
- Black body radiation
- Photoelectric effect
Lets first study about the nature of these phenomenon:
- Black body radiation: Black body is defined as perfect emitter and absorber of light.
For example, whenever we heat an Iron ball like objects, on heating they become first Red, then Orange, then Yellow and at very high temperature they become White.

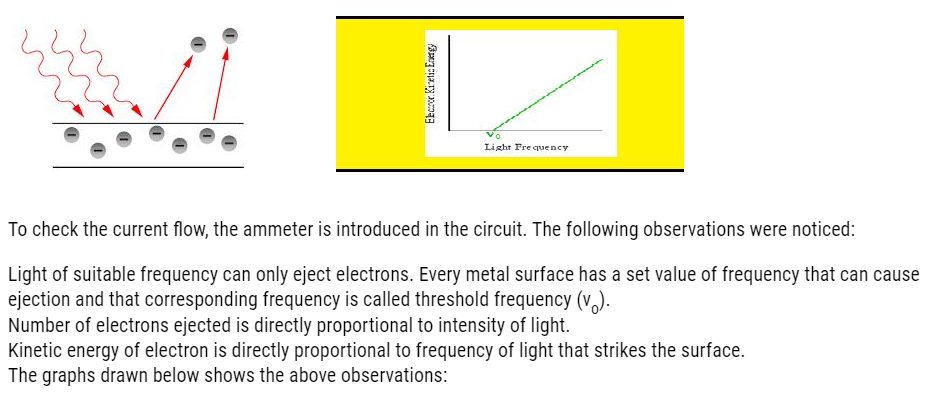
- Photoelectric effect
It is the ejection of electrons from metal surface when light of suitable wavelength strikes the metal surface.
The apparatus set to demonstrate this effect is given below: