EXPLANATION OF HYDROGEN SPECTRA
According to Bohr,
- When energy is supplied to atoms of hydrogen, the electron from lower energy gets excited to higher energy level.
- The excited state being unstable, it jumps back to its original state that is ground state.
- Some electrons move to their ground state in one jump, some in multiple jumps. Each jump corresponds to line in a spectrum.
- As we know the gas in tube consists of many hydrogen atoms.
- Therefore, each electron on getting energy gets excited.
- On returning to the ground state, they either move in single jump or multiple jump.
- This is the reason that we get so many lines in different regions in hydrogen spectrum.
The wavelength emitted by them can be calculated as:





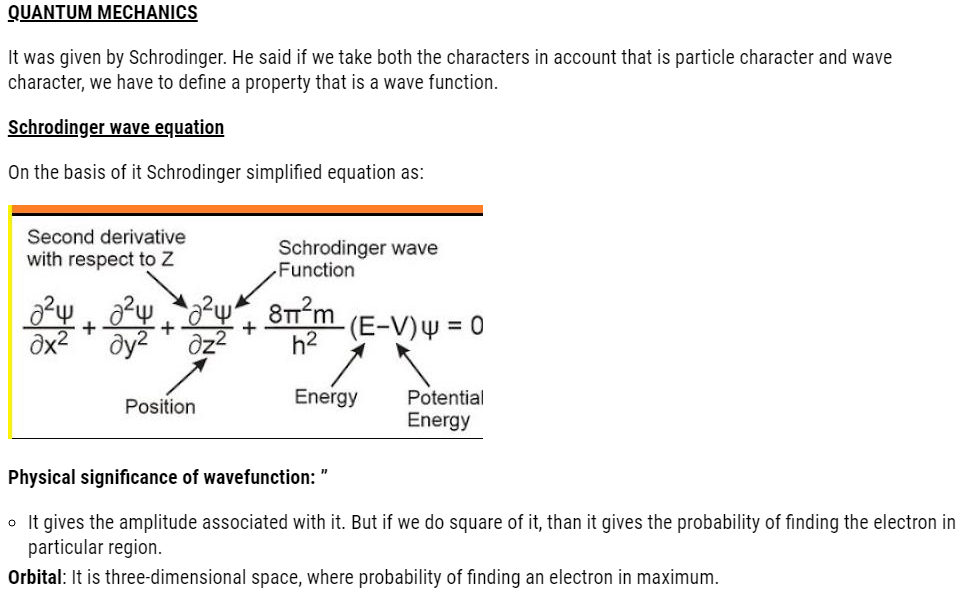
Significance
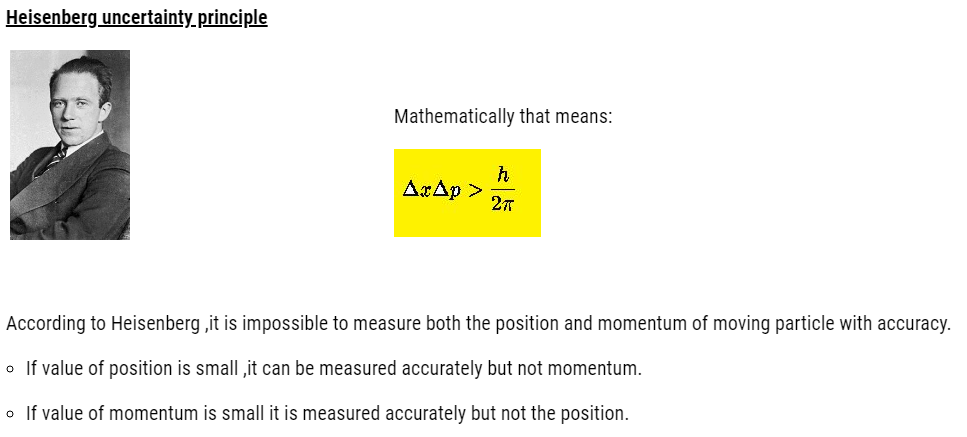
This holds good only for microscopic particles, as energy of photon is not enough to change the position and velocity of bigger bodies. So, in our daily routine it has no significance.

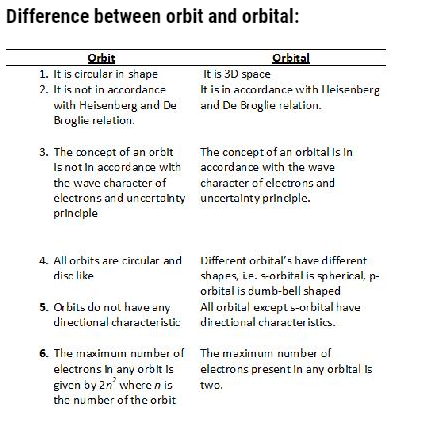
Orbital: They are of four types
- s orbital: Spherical in shape, non-directional. It has only 1 orbital therefore, can accommodate only 2 electrons.
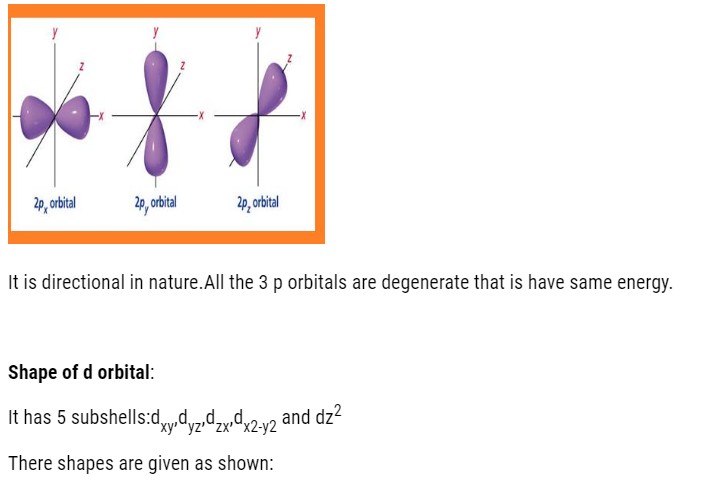
- P-orbital: dumb-bell shaped and directional. It has 3 orbital (px, py, pz). It can accommodate maximum of 6 electrons.
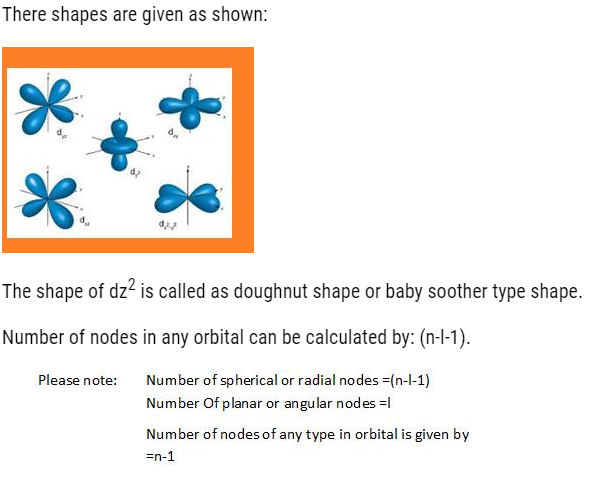
- d-orbital: It has double dumbbell, directional. It has 5 orbital (dxy,dyz,dzx,dx2-y2,dz2).It can accommodate maximum of 10 electrons
- F-orbital: It has diffused shape. It has 7 orbital therefore, can accommodate maximum of 14 electrons.
Quantum numbers
They are set of 4 numbers, which give complete information about the address of electron.
There are 4 types of quantum numbers:
- Principal quantum number.
- Azimuthal quantum number.
- Magnetic quantum number.
- Spin quantum number.
Principal quantum number:
- It is represented as ‘n’.
- It was given by Bohr.
- It represents the orbit where electron is going to be present.
Uses:
- It gives number of electron in orbit by formula 2 n2.
- It gives angular momentum of electron.
- It gives energy of electron.
- It gives radius of orbit.
- Azimuthal quantum number:
- It gives information about sub shell of an atom.
- It is represented as ‘l’.
- It was introduced by Somerfield.
- It always has value (n-1).
Example: if n=1 ,l=0
If n=2,l=0,1
If n=3,l=0,1,2
- Magnetic quantum number:
- It describes the behavior of electron in magnetic field.
- It is represented as ‘m’.
- It was given by land.
- Its value is equal to –l,0, +l
For example: if n=1, l=0, m=0 that is only one orbital
If n=2, l=0,1, m=-1,0, +1 that is three orbitals
- Spin quantum number:
- It gives the info about spinning of electron about its axis i.e. clockwise or anticlockwise
- It is denoted by ‘s’.
- Its value is either =+1/2, -1/2
Problem: Write down the quantum numbers n , l and m for the
Following orbitals:
- 3d x2 – y2
- 4d z2
Answer :-
n=3, l=2, m=+2
N = 4, l=2, m=0
Pauli’s exclusion Principle
According to it: “no two electrons can have the same set of all four quantum numbers.”
Or, it states that an orbital can have maximum of two electrons and that must be of opposite spin. Due to this, it was concluded that an orbital can have maximum of two electrons which can have all 3-quantum number same but the spin will be definitely different.
Shapes of atomic orbitals:
- s –orbital
Its shape is spherical.For 1s the probability of finding electron is maximum near nucleus and it decreases as we move away from nucleus.
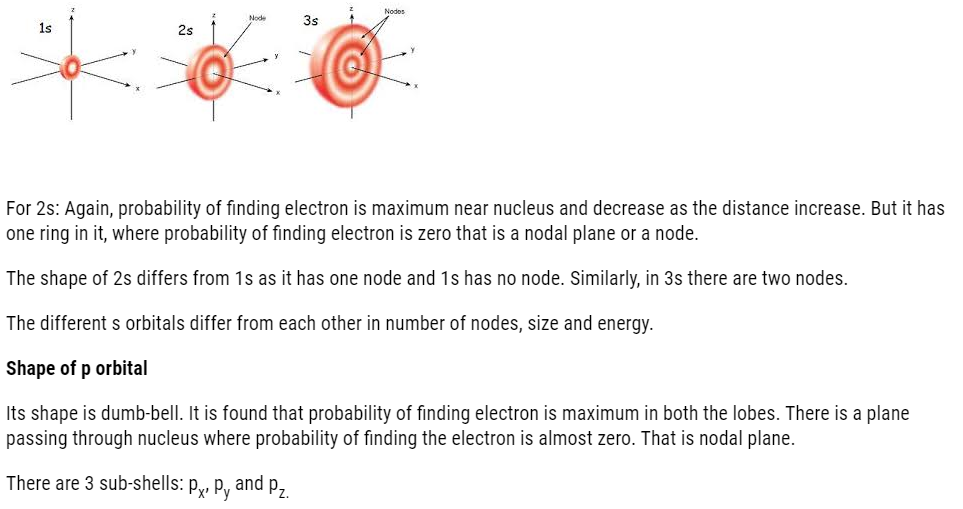

1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p…
To know the energy of orbital with lower energy the n+l rule is followed:
According to it:
- The lower the value of n+l for an orbital, the lower is its energy and is filled first.
For example: Out of 2s and 2p ,2s is filled first as n+l for 2s, is 2+0=2 and for 2p it is 2+1=3. Therefore, 2s is filled first.
- If two orbitals have same value of n+l , than the orbital with lower value of n is filled first.
For example: Out of 3p and 4s, n+l for both is 4 . Therefore, 3p is filled first as it possess lesser value of n.
- Pauli’s exclusion principle: According to it, an orbital can accommodate maximum of two electros and that must be of opposite spin.
For example: If orbital has s2, then the orbital has the arrangement as given below.

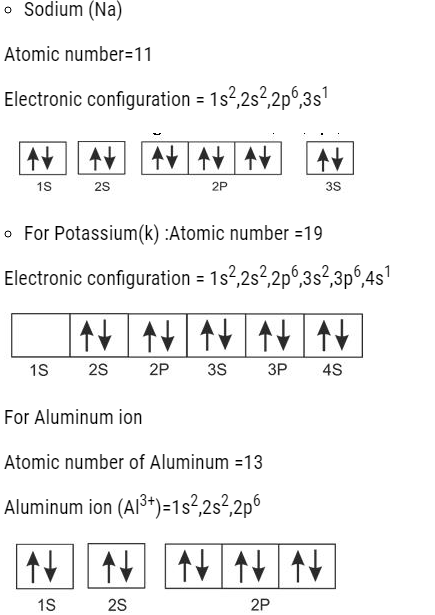
EXCEPTIONAL ELECTRONIC CONFIGURATION OF CHROMIUM AND COPPER
For Copper (Cu)= [Ar]18,4s23d9
But actually, it has : [Ar]18,4s13d10
Similarly, for Chromium it should be: [Ar]18,4s23d4
But in actual it is:=[Ar]18,4s13d5
The reason behind is:
- Half-filled and fully filled orbital are more stable
If configuration is 4s2 3d9 , then the d orbital is not fully filled. If configuration is 4s1 3d10 , then the d orbital is completely filled. That means it becomes more stable.
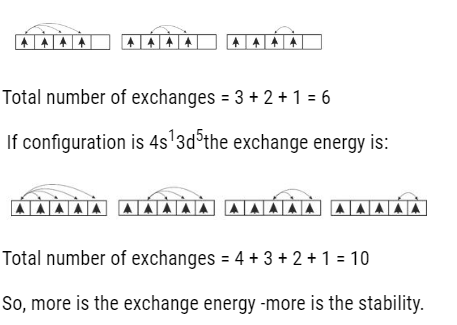
- The more is the exchange energy more stable is the orbital as shown below:
If configuration was 4s23d4 the exchange energy is: