MORE QUESTIONS SOLVED
I. Very Short Answer Type Questions
Question 1. How is bond order related to the stability of a molecule?
Answer: Higher the bond order, greater is the stability.
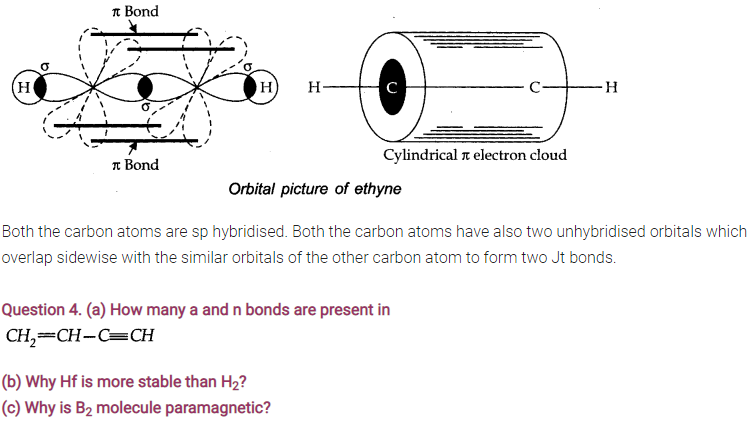
Question 2. Write the type of hybridisation involved in CH4,C2H4 and C2H2.
Answer: CH4= sp3
C2H4 = sp2
C2H2 = sp
Question 3. Out of sigma and Π bonds, which one is stronger and why?
Answer: sigma-bond is stronger. This is because sigma-bond is formed by head-on overlapping of atomic orbitals and Π bond is formed by side wise overlapping.
Question 4. Write the Lewis dot symbols of the following elements and predict their valencies. (i) Cl (ii) P
Answer:

Question 8. What is meant by bond pairs of electrons?
Answer: The electron pairs involved in the bond formation are known as bond pairs or shared pairs.
Question 9. Which of the following has larger bond angle in each pair?
(i) CO2, BF3 (ii) NH3, CH4
Answer: (i) CO2 (ii) CH4
Question 10. Arrange the following, according to increasing covalent nature.
NaCl, MgCl2, AlCl3
Answer: NaCl < MgCl2 < AlCl3
Question 11. Define covalent bond according to orbital concept?
Answer: Covalent bond can be formed by the overlap of the orbitals belonging to the two atoms having opposite spins of electrons.

Question 15. State the types of hybrid orbitals associated with (i) P in PCl5 and (ii) S in SF6
Answer: (i) sp3d of P in PCl5 (ii) sp3d2 of S in SF6
Question 16. Why N2 is more stable than O2? Explain on the basis of molecular orbital theory.
Answer: Bond order of N2 (= 3) is greater than that of O2 (= 2).
Question 17. How is bond order related to bond length of a molecule?
Answer: Bond length is inversely proportional to bond order.
Question 18. Out of bonding and antibonding molecular orbitals, which one has lower energy and which one has higher stability?
Answer: Bonding molecular orbital has lower energy and higher stability.
Question 19. Define antibonding molecular orbital.
Answer: The molecular orbital formed by the subtractive effect of the electron waves of the combining atomic orbitals, is called antibonding molecular orbital.
Question 20. Name the two conditions which must be satisfied for hydrogen bonding to take place in a molecule.
Answer: (i) The molecule should contain highly electronegative atom like hydrogen atom. (ii) The size of electronegative atom should be small.
II. Short Answer Type Questions
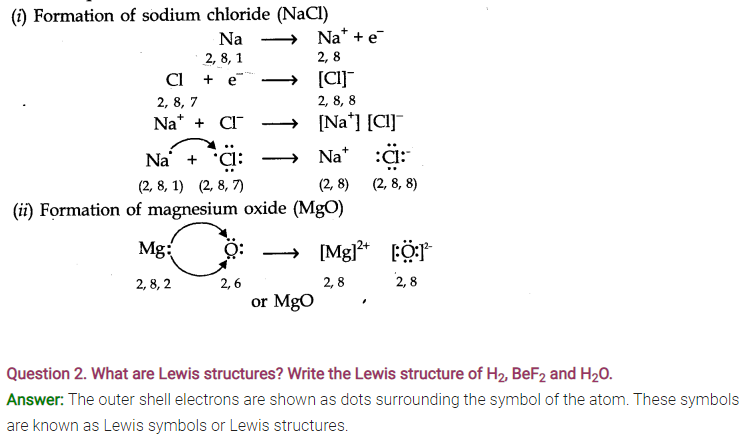
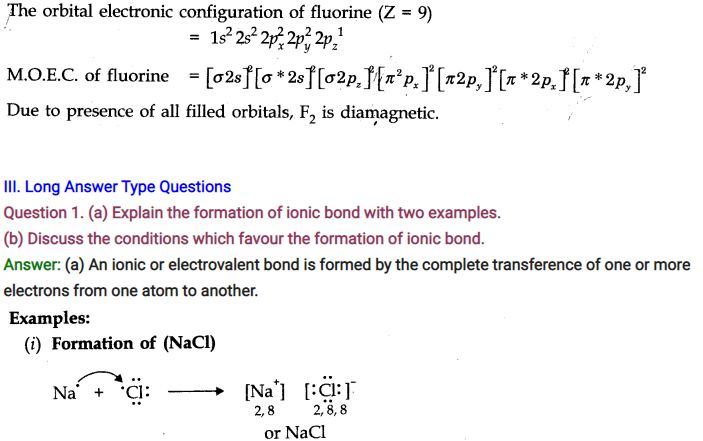
Question 1.What is an electrovalent (or ionic) bond? Explain its formation with two examples.
Answer: When a chemical bond is formed by the complete transfer of electrons from one atom to another, so as to complete their outermost shell and therefore, aquire the stable noble gas configuration, the bond formed is called ionic bond or electrovalent bond. ‘
For Example,



Question 8. What are the main postulates of Valence Shell Electron Pair Repulsion (VSEPR) theory?
Answer:
- The shape of a molecule depends upon the no. of electron pairs around the central atom.
- There is a repulsive force between the electron pairs, which tend to repel one another.
- The electron pairs in space tend to occupy such positions that they are at maximum distance so, that the repulsive force will be minimum.
- A multiple bond is treated as if it is single bond and the remaining electron pairs which constitute the bond may be regarded as single super pair.
Question 9. Define bond order. How is it related to the stability of a molecule?
Answer: Bond order is defined as half of the difference between the number of electrons present in bonding and antibonding molecular orbitals.
Bond order (B.O.) = 1/2[Nb – Na ] z
If the bond order is positive (Nb > Na), the molecule or ion will be stable. If it is negative (Nb < Na)the molecule or ion will be unstable.
Question 10. Explain the diamagnetic behaviour of P2 molecule on the basis of molecular orbital theory.
Answer:

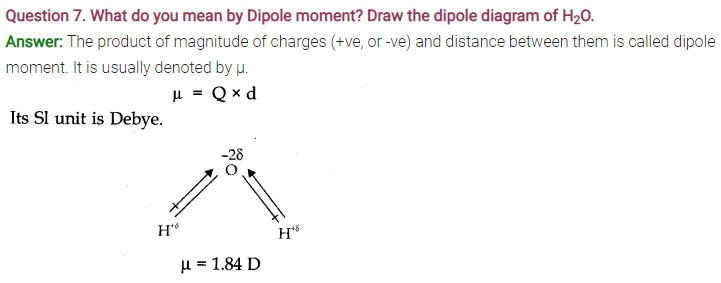
Question 2. (a) Define dipole moment. What are the units of dipole moment?
(b) Dipole moment values help in predicting the shapes of covalent molecules. Explain.
Answer: (a)Dipole moment: In a polar molecule, one end bears a positive charge and the other has a negative charge. Thus, the molecule has two poles with equal magnitude of the charges. The molecule is known as dipolar molecule and possesses dipole moment.
It is defined as the product of the magnitude of the positive or negative charge and the distance between the charges. µ (dipole moment) = q x d
SI unit of dipole moment is coulomb metre (m) or Debye.
(b)The dipole moment values are quite helpful in determining the general shapes of molecules.
For molecules with zero dipole moment, shapes will be either linear or symmetrical. For Example. BeF2 CO2etc. Molecules that possess dipole moments, their shape will not be symmetrical.
Question 3. Discuss the orbital structures of the following molecules on the basis of hybridisation, (i) BH3 (ii) C2H2
Answer: