NCERT Solutions for Class 11 Chemistry Chapter 13 Very Short Answer Type Questions
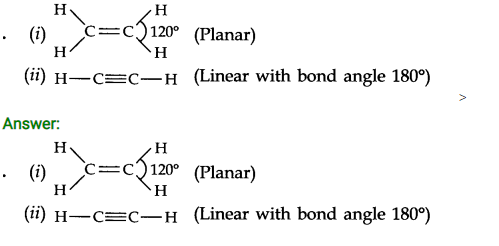
Question 1.
Question 2. What are conformations?
Answer: Conformations are spatial arrangements which are obtained by rotation around sigma bonds.
Question 3. What is decarboxylation ? Give an example.
Answer: The process by which carbon dioxide is removed from sodium acetate (or any sodium salt of acid) with the help of sodalime is called decarboxylation.

Question 4. What do you mean by pyrolysis?
Answer: The decomposition of a compound by heat is called pyrolysis. This process when applied to alkanes is known as cracking.
Question 5. What happens when ethanol is heated with cone. H2SO4?
Answer:
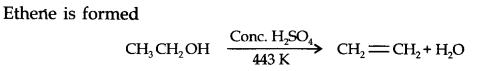


Question 10. Arrange the three isomers of pentane in increasing order of their boiling points.
Answer: 2, 2-Dimethylpropane < 2-methylbutane < pentane.
Question 11. Arrange the following: HCl, HBr, HI, HF in order of decreasing reactivity towards alkenes.
Answer: HI > HBr > HCl > HF
Question 12. Although benzene is highly unsaturated it does not undergo addition reactions. Why?
Answer: It is due to delocalization of π -electrons in benzene it is highly stable.
Question 13. Why are Alkenes called olefins?
Answer: Alkenes are commonly known as olefins because the lower members form oily products on treatment with chlorine or bromine.
Question 14. Which is more acidic: ethene or ethyne and why?
Answer: Ethyne is more acidic than ethene because it has ‘sp’ hybridised ‘C’ which is more electronegative.
Question 15. What is Huckel rule?
Answer: Huckel rule states that a compound is said to be aromatic if it has (4n + 2) n electrons
delocalized where n = an integer 0,1, 2, 3,
Question 16. How will you distinguish between propene and propane?
Answer: Pass them through dilute cold KMnO4solution (purple) or Br2 in CCl4 solution (red). Propene will decolourise both the solutions but propane does not react.
Question 17. How will you distinguish between acetylene and ethylene?
Answer: Acetylene forms precipitate with ammoniacal silver nitrate solution, ethylene does not react with these reagents.
Question 18. What happens when benzene is treated with acetyl chloride in presence of AlCl3?
Answer: Acetophenone is formed.
Question 19. Which type of isomerism is exhibited by but-l-yne and but-2-yne?
Answer: Position isomerism.
Question 20. What is electrophile in sulphonation?
Answer: SO3.
Question 21. What is the hybridisation of central carbon in 1,2-propadiene (CH2=C=CH2)?
Answer: sp.
Question 22. What are Arenes?
Answer: Arenes are aromatic hydrocarbons.
NCERT Solutions for Class 11 Chemistry Chapter 13 Short Answer Type Questions
Question 1.Define resonance energy. What is resonance energy of benzene?
Answer: Resonance .energy is the difference in energy between actual structure of compound . and most stable resonating structure. The resonance energy of benzene is 150.325 J mol-1.
Question 2. Explain the following with examples:
(i) Wurtz reaction
(ii) Hydrogenation.
Answer: (i) Wurtz reaction: Alkanes are produced by heating an alkyl halide with sodium metal in dry ether solution.

Question 4. Classify the following compounds into (i) alkanes (ii) alkenes (iii) alkynes (iv) arenes. (a) C6H6 (b) C4H8 (C) C8H8 (d) C5H8 (e) C6H14
Answer: (i)Alkanes — C6H14, C8H18
(ii)Alkenes — C4H8
(iii)Alkynes — C5H8
(iv)Arenes — C6H6.
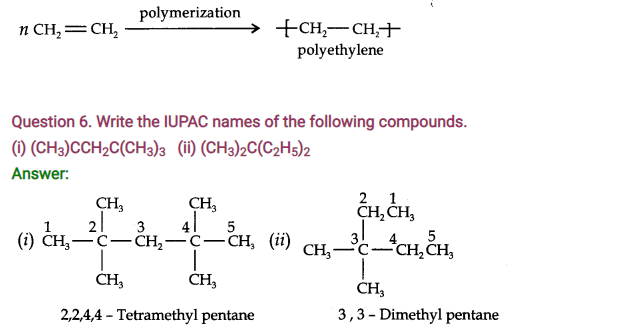
Question 5. What is polymerization? Give an example.
Answer: The process by which simple molecules join together to form large molecules is known as polymerization.
Simple alkenes polymerize to form long chain addition polymers.
For example, ethylene gives polyethylene.
Question 7. Write the structure and IUPAC names of different structural isomers of alkenes corresponding to C5H10.
Answer:

Question 8. Draw the structures of cis- and trans-isomers of the following compounds. Also write their IUPAC names.(i) CHCl—CHCl (ii) C2HC(CH3)=C(CH3)C2H5
Answer:

Question 9. (a) What effect the branching of an alkane has on its melting point?
(b) Which of the following has highest boiling point?
(i) 2-methyl pentane
(ii) 2, 3-diethyl butane
(iii) 2, 2-dimethyl butane
Answer: (a) In general conception, as the branching increases packing of the molecules in the crystal lattices becomes less close and hence melting point decreases accordingly. (b) As the branching increases, surface area decrease and thus magnitude of van der Waals forces of attraction decreases and hence the boiling point decreases. 2,2-dimethyl butane has lower surface area due to more branching and hence has lower boiling point.

NCERT Solutions for Class 11 Chemistry Chapter 13 Long Answer Type Questions
Question 1. Explain the term aromaticity. What are the necessary conditions for any compound to show aromaticity?
Answer: The aromatic compounds apparently contain alternate double and single bonds in a cyclic structure, and resemble benzene in chemical behaviour. They undergo substitution reactions rather addition reactions. This characteristic behaviour is called Aromatic character or Aromaticity.
Conditions for Aromaticity:
- An aromatic compound is cyclic and planar.
- Each atom in an aromatic ring has a p-orbital. These p-orbitals must be parallel so that a continuous overlap is possible around a ring.
- The cyclic n-molecular orbital formed by the overlap of p-orbitals must contain (4n + 2) K electrons, where (n = 0,1, 2, 3, 4 etc.)
Question 2.(a) Define substitution reactions. Why do benzene undergo substitution reactions even though they contain double bonds?
(b) What happens when benzene is treated with
(i) Br2 in presence of anhydrous AlCl3
(ii) Cone. H2SO4 at 330K
(iii) Mixture of cone. H2SO4and com. HNO3 at 330 K
(iv) Ethanoyl Chloride in presence of anhydrous AlCl3
Answer: (a) Substitution reactions are those reactions in which an atom or group of atoms directly attached to carbon in the substrate molecule is replaced by another atom or group of atoms, for example,

A hydrogen atom of the methane molecule is replaced by chlorine atom. Benzene undergoes electrophilic substitution reactions because benzene ring has delocalized electrons is an electron-rich system. It is attacked by electrophiles giving substitution products.

Question 3. (a) What type of isomerism is shown by methoxymethane and ethanol?
(b) How will you bring out the following conversions.
(i) Acetylene to ethane (ii) Benzene to Toluene (iii) Ethanol to ethene?
Answer: (a) Functional isomerism.

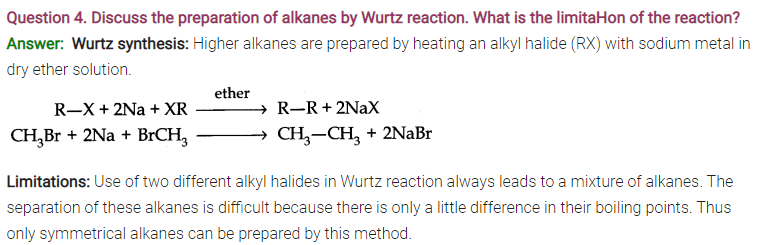
Question 5. (a) Why are alkenes called unsaturated hydrocarbons?
(b) How will you test the presence of double bond in an alkene?
(c) Name the products formed when propene is subjected to ozonolysis.
Answer: (a) Alkenes contain two hydrogen atoms less than alkanes and thus they contain C—C double bond (C=C) in their molecule. Thus they are called unsaturated hydrocarbons.
(b) Alkenes react with cold dilute KMn04 solution to form gycols. Since bright purple colour of KMn04 disappears during the reaction it is used as a test for the presence of double bond.
(c) A mixture of acetaldehyde and formaldehyde is formed.