Preparation of washing soda by solvay’s process
- The Solvay process is extensively used for industrial preparation of sodium carbonate (soda ash). The process is named after Ernest Solvay who developed the process during the 1860s.
- Carbon dioxide reacts with the dissolved ammonia to form ammonium carbonate followed by ammonium hydrogen carbonate.
2NH3 +H2O + CO2 —> (NH4)2CO3
(Ammonia) (Ammonium Carbonate)
(NH4)2CO3 + H2O + CO2 –> NH4HCO3
(Ammonium Carbonate) (Ammonium hydrogen carbonate)
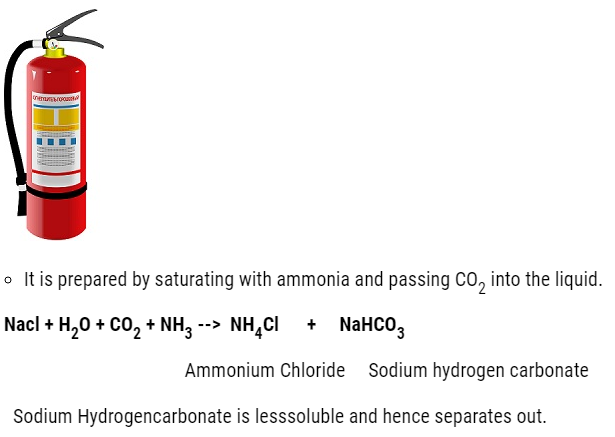
NH4HCO3 + NaCl –> NH4Cl + NaHCO3
Sodium hydrogen carbonate crystal separates. These are heated to give sodium carbonate.
2 NaHCO3 –> Na2CO3 + CO2 + H2O
But this process cannot be used to prepare potassium due to the high solubility of potassium bicarbonate and hence difficult to be precipitated by adding ammonium bicarbonate to a saturated solution of potassium

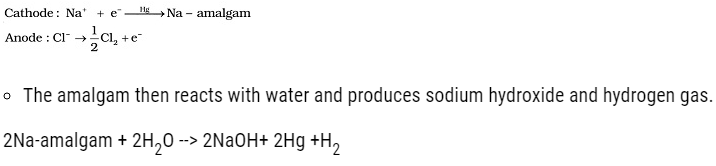
Caustic Soda
- The chemical name of caustic soda is Sodium Hydroxide (NaOH).



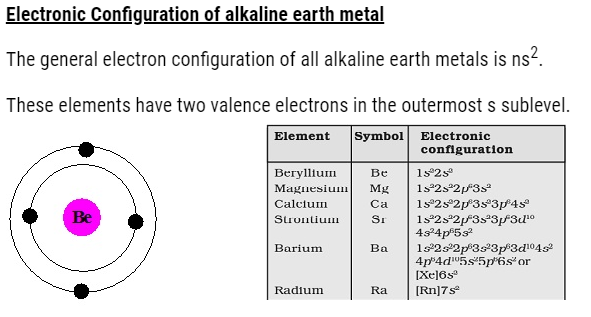
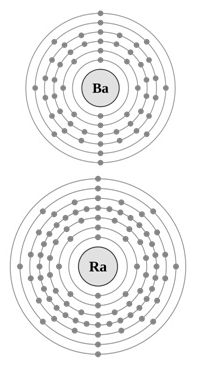
Atomic and Ionic Radii of alkaline earth metal
- The atomic and ionic radii of the alkaline earth metals are smaller than alkali metals due to the increased nuclearcharge in these elements.
- Theatomic and ionic radii increase with increase in atomic number within a group.