Quick Lime
- The chemical name of Quick Lime is Calcium Oxide (CaO).

- It is a white amorphous solid.
- The melting point of CaO is 2870 K.
- It absorbs moisture and carbon dioxide when exposed to atmosphere.
- It is used in manufacturing industry to manufacture cement, dye stuffs and sodium carbonate.
- It is also used for the purification of sugar.
- It is prepared byheating limestone (CaCO3) in a kiln at a temperature of 1070-1270 K.
CaCO3 <–> CaO + CO2
- Addition of water to CaO results in slaking of lime.
- Slaking of Quick lime with sodaresults solid soda lime.
- CaO being a basic oxide combines with acidic oxides at high
CaO + SiO2 –> CaSiO3
6CaO + P4O10 –> 2Ca3 (PO4)2

Calcium Carbonate (CaCO3)
- It is a white fluffy powder that is insoluble in water and gets decomposed followed by the release of carbon-dioxide on heating to 1200 K.
CaCO3 + 1200k –> CaO + CO2
CaCO3 + 2HCl –> CaCl2 + H2O + CO2
CaCO3 + H2SO4 –> CaSO4 + H2O + CO2


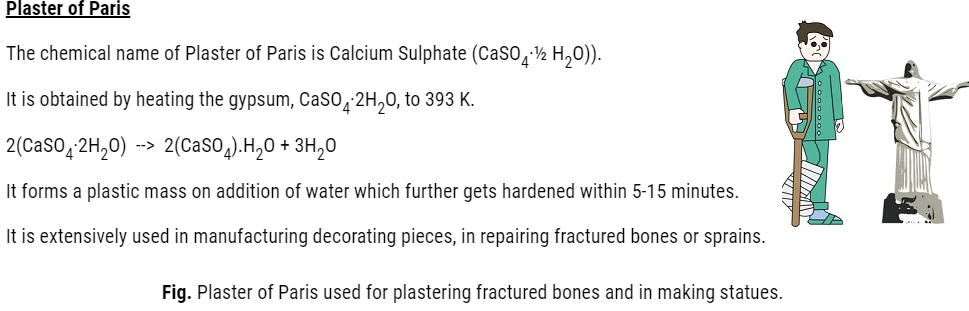
Cement
- Combination of CaO with clay containing silica, SiO2 along with the oxides of aluminium, iron and magnesium leads to the formation of cement.
Composition of Portland cement
- CaO = 50-60%;
- SiO2 = 20-25%;
- Al2O3 = 5-10%;
- MgO = 2-3%;
- Fe2O3 = 1-2%
- SO3 = 1-2%.
Strong heating of clay andlime results in their fusion followed by the formation of cement clinker which is then mixed with 2-3% by weight of gypsum(CaSO42H2O). This leads to the formation of cement.
- Important ingredients of Cement:
Dicalcium silicate (Ca2SiO4) = 26%,
Tricalcium silicate (Ca3SiO5) = 51%
Tricalcium aluminate (Ca3Al2O6) = 11%
- Settling of cement:
Addition of water to cement hydrates the molecules of the constituents. Gypsum is added in order to slow down the process of setting of the cement to make it get hardened.
- It is used to prepare concrete in plastering and constructingbridges, dams and buildings.

- Many fertilizers are manufactured using potassium
- Sodium in its liquid state is used as a coolant infirm breeder nuclear reactors.
- Beryllium is used for creating alloys.
- Beryllium in combination with copper is sued to create strong springs.
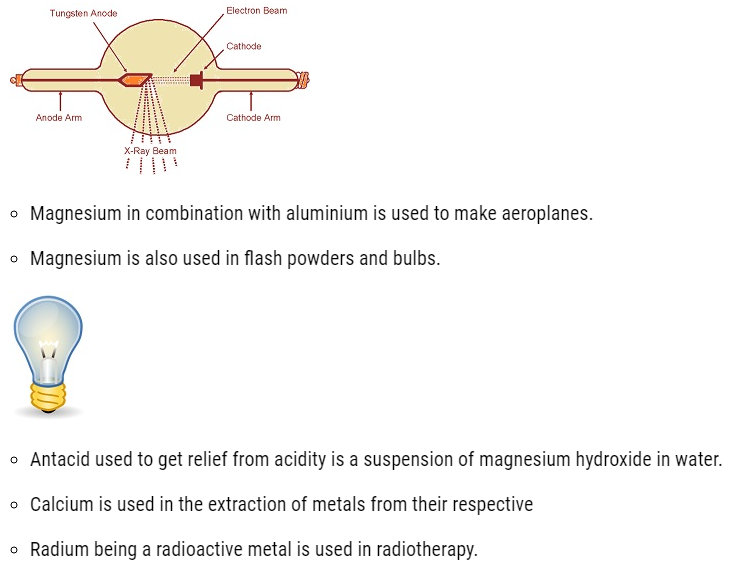
- Beryllium is used for making windows ofX-ray tubes.


Question: Write balanced equations for reactions between
(a) Na2O2 and water
(b) KO2 and water
(c) Na2O and CO2
Answer:
(a) 2 Na2O2(s) + 2 H2O(l) –> 4 NaOH(aq) + O2(g)
(b) 20 KO2 + H20 –> 20 KOH + 10 O2
(c) 2 Na2O + 3 Co2 –> 2 Na2Co3 + O2