Redox couple
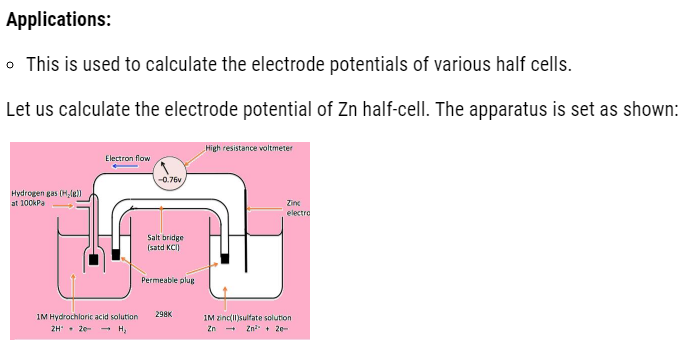
It is defined as having together the oxidized and reduced forms of a substance taking part in oxidation or reduction half reactions .they are actually conjugate acid base pairs .we can also define it as : an oxidizing and reducing agent which appear on opposite sides of a half equation constitute the redox couple .Like, given below is the cell in which we consider Zn/Cu redox couple .
Electrochemical cell
- Electrochemical cell is the cell in which chemical energy gets converted to electric energy.
- In it indirect redox reactions takes place.
- These reactions are spontaneous that is free energy change for this reaction is negative.
- This cell consists of two half cells.
- In one half cell , there is a aqueous 1molar Zinc sulphate solution with Zinc rod dipped in it.
- In other half cell, there is a 1 molar aqueous solution of Copper sulphate solution with Copper rod dipped in it.

Observations
- With time we see that Zinc rod loses weight, as it has more tendency to loose electrons that is:
Zn -2 electrons –> Zn2+ (Oxidation)
Zinc Zinc Ion
- These electrons released by zinc, travel to another beaker by means of wire. In doing so, they cause deflection in galvanometer and produce current. This current travel in the direction opposite to the flow of electrons.
- These electrons move to another half cell, where copper ions gain these electrons that is reduction occur. As a result, copper metal start depositing on electrode. The reaction that occurs is shown below:
Cu2+ + 2electrons –> Cu(reduction)
Copper ions Copper Metal
Functions of salt bridge
- It connects the circuit internally by connecting the solutions.
- It helps in maintain neutrality.
With passage of time, the left container will have excess positive charge around electrode. Due to which further oxidation stops .Whereas in other beaker negative charge will exceeds, which will start repelling electrons. Therefore, at that time salt bridge comes into action. The oppositely charged electrolyte ions start diffusing into half cells in order to neutralize the excess charge. Hence, the cell keeps on working.
The electrolyte that is selected must fulfill two conditions:
- Size of its cation and anion should be equal.
- Electrolyte in salt bridge should not interact with the main electrolyte of half cells.
The overall reaction that takes place is:
Zn + Cu2+ –> Zn2+ + Cu
Zinc Copper Zinc Ion Copper Metal
Representation of the cell

Electrode potential
Electrode potential is defined as “potential difference set up between electrode and electrolyte of same beaker”.
It is of two types:
- Reduction potential: Tendency of solution to get reduced.
- Oxidation potential: Tendency of electrode to get oxidized.
Factors on which electrode potential depends:
- Concentration of ions in solution.
- Nature of metal and its ions.
Electromotive force
It is the potential difference between two electrodes when no current flows through the circuit.
Standard hydrogen electrode and its application
Standard hydrogen electrode is the reference electrode that is used to calculate electrode potential of any electrode. It is also called as SHE or NHE.
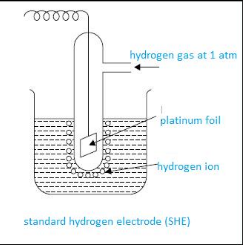
This apparatus consists of beaker having 1 molar HCL solution. In it, a sealed tube having platinum wire is dipped. This platinum wire is further attached to platinum foil. This complete cell is connected to the cell. Then continuously hydrogen gas maintained at 1 atm is bubbled. Platinum foil here acts as a site of reaction.
The Standard electrode potential of SHE is zero volt. This SHE can act as anode or cathode, depending upon the half cell that is attached to it.
If it acts as cathode then following reaction occurs:
2H+ + 2e– –> H2 (Reduction Occurs)
Hydrogen Ion Hydrogen Gas
If it acts as anode then following reaction occur:
2H – 2e– –> 2H+ (Oxidation occurs)
Hydrogen Gas Hydrogen Ions

Electrochemical series
Is the arrangement of elements in order of increasing potential. It is the series has the values starting from –ve to positive. The series is shown below:
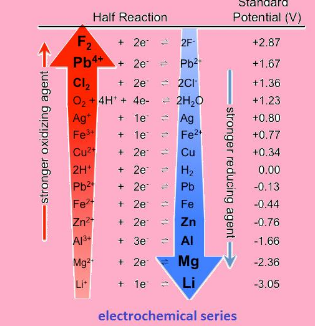
Application:
- The series tells us about the strength of reducing or oxidizing agents
“Lower the value of potential, stronger is the reducing agent “or vice versa.
- The series tells us how to calculate standard electrode potential for cell that is :
E* = (Ec – Ea)
- The series helps us to predict the feasibility of reaction.
- ‘If value of E* is positive , then the reaction is feasible
- If not positive, than the given reaction is not feasible “
- The series helps us to know which metal will evolve hydrogen gas and which will not.
“All the metals with negative reduction potential will evolve hydrogen and others do not.”
Redox reaction as the basis for Titrations
Titration is the process in which the solutions of two reagents are allowed to react with each other.
Procedure:
- In it one solution (known volume) is taken in Burette and the solution is called titrant.
- The other reagent is taken in flask called titration flask and the solution is called as analyte.
- The titration is carried out till both the reagents mix completely.
- The stage at which both the reagent mix completely is called end point.
- The end point is detected by indicator.
The objective of these titrations is to find out the exact amount of an acid (or the base) present in a given solution by reacting it against the solution of standard base (or an acid) .
Introduction
We have studied about so many elements till now .Out of 114 elements, most of them occur in combined state. Only noble gases occur in atomic state otherwise, most of them exist in molecular or combined form.
The combined state is formed by combination of elements that is a chemical bond. Like families, have connections in the same way the attraction of chemical species is called chemical bond.
The reason behind the formation of chemical bond is to obey octet rule .According to this rule, “every element needs to attain stability. For this, the elements either loose or gain electrons to attain 8 electrons in its outermost shell” .But certain violation were seen like in case of hydrogen, the atom has tendency to attain only 2 electrons in valence shell .So, in case of it the rule is violated and is called as Duplet rule.
Other violations are: In certain molecules, the deficient octet is seen. For example in H2 ,whereas in others the central atom has expanded octet that is more than 8 electrons like in SF6.
When two atoms come closer, their outer electrons repel each other and finally they stop at a distance where the released energy is more .As less energy corresponds to maximum stability than at that distance the chemical bond is formed.
Bond formation takes place due to loss of energy and stable state.